User:Andy Maloney/Notebook/Lab Notebook of Andy Maloney/2010/03/08/Fixed microtubules
Fixed microtubules...Attempt 1
In order to fix microtubules to a glass slides, we use poly-L-lysine. A previous attempt at getting fixed microtubules failed due to bad Taxol and possibly due to using too much poly-L-lysine.
Fixed microtubules...Attempt 2
I had two goals with this. One, to look at fixed MTs with poly-L-lysine and to look at MTs with different amounts of fluorescent labeling for Larry's paper. Here's what I did:
- I took 2 aliquots of unlabeled tubulin and 1 aliquot of rhodamine labeled tubulin out of the -80 freezer. I took one unlabeled aliquot and removed 1 uL from it. I then added the rhodamine tubulin for a solution that has 20% labeled tubulin.
- I then aliquoted the 20% labeled tubulin and took an extra 1uL of it and aliquoted that. I then added 1uL of unlabeled tubulin for a 10% aliquot.
- I then serial diluted the tubulin for a 5% and 2.5% aliquots.
- I polymerized the microtubules at 37C for 30 minutes
- I should note that the 5% labeled tubulin assay popped open in the thermal cycler thus I didn't have a 5% labeled assay. I cleaned up the mess and moved on.
- For each slide, I incubated the slide with 1 mg/mL poly-L-lysine for 10 minutes.
- I then flushed the flow cell with 3 flow cell volumes (30uL).
- I added 10uL of motility solution and incubated upside down for another 10 minutes.
- I then flushed the cell again with 30uL. Sealed and observed.
- The microscope was setup for Kohler illumination and the field stop for the Hg lamp was increased just beyond the FOV for the camera. I used 25% Hg illumination, 100 ms exposure and a gain of 150.
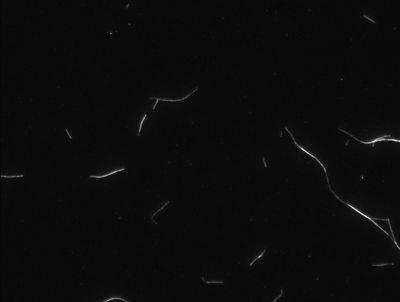
20% label
The image below show fixed MTs which have been labeled with 20% rhodamine tubulin. You can see the speckle somewhat.
One thing I don't understand is why am I getting logs? Is this because of the poly-L-lysine?
10% label
You can definitely see more speckling going on in this image.
5% label
For completeness, I should probably do this one over again.
2.5% label
In this slide I should note that I did not use PEM-T in the motility solution. I used PEM. This was totally my mistake from being distracted.
The speckling in the above image is definite. I hope these images can be used as an experimental verification for the reasoning behind why Larry chooses random locations for his emitters in his simulation. Perhaps Larry can make his microtubules with 20%, 10%, 5%, and 2.5% emitters in his simulation for comparison.
Conclusion
So there are some things that I'm not sure of with the above experiment and poly-L-lysine. Some questions include:
- Why do the MTs appear to be thinner?
- Why am I getting logging?
- Andy Maloney 19:07, 16 March 2010 (EDT): There could be free poly-L-lysine which is causing the MTs to stick together. Koch and I think this is the case since every protocol we've come across uses a lot less poly-L-lysine. Something like 50-100µg/mL. Plus, the other protocols dry out the slides that have been functionalized. It's apparent that more research must be done.
- Are the above images something close to what Emmalee and I want for her experiments?
- Steve Koch 01:40, 17 March 2010 (EDT): I think, yes, those kind of fixed MTs would be good. Probably would be good for 3 of us to talk when you're doing the experiments, to regroup.
Planning for another run
Scheduling with Emmalee will be determined either here or through email. The things that I can do on my end include:
- Preparing fluorescein microtubules.
- Determining the correct amount of poly-L-lysine to use as our "fixer".
- Andy Maloney 17:36, 16 March 2010 (EDT): This I'm still not too sure on. Sigma states that you can use 0.01% mg/mL poly-L-lysine as a surface blocker but in the above second attempt, I used 1 mg/mL just fine. I think that in attempt 1 we used 5 mg/mL and we got clumps. I also didn't wash out the poly-L-lysine in the first attempt.
- Ensuring that we can fix microtubules reproducibly.
- Andy Maloney 17:36, 16 March 2010 (EDT): Yes, we can fix microtubules reproducibly.
Experiment
Emmalee's Notes:
In the past weeks, I have made lipid vesicles using both straight PC lipid and a combination of PC and PG with Texas Red that I will use for the experiments with MTs. I was able to use dynamic light scattering to find the size of the vesicles to be around 100 nm. (Hydronamic radius varied from 50-70 nm)
Unfortunately, while I was using the mini-extruder to make my last batch of vesicles, the end of one of the syringes broke off. I have ordered a replacement, and these are the steps I would like to take once it arrives.
- Visualize MT and vesicles on polylysine surface (I can bring over a Texas Red cube for the microscope)
- Introduce tau onto surface with MT and vesicles and observe any change in binding to MT
- Use physiological and mutant hyperphosphorylated tau
I asked for rush shipping on the syringe, so hopefully it will get here before the end of the week. After it arrives, I will need a few hours to prepare new vesicles and confirm their size with DLS.
- Andy Maloney 19:13, 16 March 2010 (EDT): If you are using the hand extruder from Avanti, we have one in our lab as well in case the syringe breaks again.
I anticipate being able to work with the vesicles Friday (Mar 12). At the latest, I should have them ready the next Monday (Mar 15).