User:Andy Maloney/Notebook/Lab Notebook of Andy Maloney/2009/08/27/Kinesin walking
Today Larry and I decided to go over some simple kinesin stepping ideas. I'm still far behind him when it comes to the literature so I need to catch up at some point.
I am going to basically rehash what he has done already in my own words. This is to benefit me and no one else.
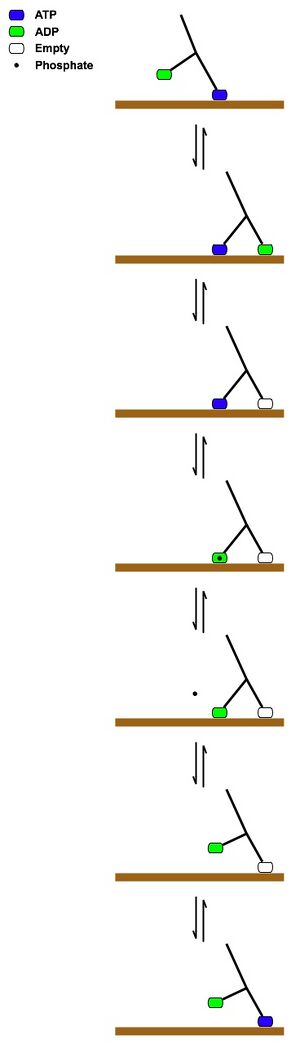
The picture above describes what people think is going on when a kinesin molecule walks along a microtubule. Basically what is happening is:
[math]\displaystyle{ \begin{align} K_1\cdot ATP\cdot MT+K_2\cdot ADP&\leftrightharpoons K_1\cdot ATP\cdot MT+K_2\cdot ADP\cdot MT\\ K_1\cdot ATP\cdot MT+K_2\cdot MT &\leftrightharpoons K_1\cdot ADP\cdot MT\cdot P_i+K_2\cdot MT\\ K_1\cdot ADP\cdot MT+K_2\cdot MT+P_i&\leftrightharpoons K_1\cdot ADP+K_2\cdot MT\\ &\leftrightharpoons K_1\cdot ADP+K_2\cdot ATP\cdot MT \end{align} }[/math]
And then it repeats the process. Of course each reaction has associated rate constants to them and we do know some of them. Not only do the 7 steps mentioned above occur but, more "non regular" steps can occur (for each step) that can either slow down the process or inevitably stop the processivity.
Constraints
Larry and I also hashed out some constraints that think kinesin may have. They include:
- When a foot has an ATP bound to it, this configuration has a stronger binding to the microtubule than a foot that has an ADP bound to it.
- If a kinesin is not bound to a microtubule, then it more than likely has an ADP bound to it.
- There is a very low chance that ATP will be bound to both feet.
- Block states that the kinesin foot spends 93% of its time unbound to the microtubule if it is under a load.
- 1:1 ratio of ATP used for each step.
- A kinesin molecule takes approximately 100 steps before detaching from a microtubule.
- Kinesin's travel time is about 1 second.
- The kinesin's velocity follows Michaelis-Menten kinetics.
Things to look up
We also came up with a slew of things to look up. Each one encompasses an idea that we have about the way kinesin walks.
- Is there a difference in the crystal structure of a kinesin foot when it has ATP or ADP bound?
- How do microtubule residues interact with kinesin's feet?
- How ATP and the "power stroke" are related.
- Does ATP interact with the residues on microtubules? If so, how.
- Is strain real? I.e. the strain felt in kinesin's legs.
- What is the persistence length for the legs of kinesin?