User:Andy Maloney/Kinesin & Microtubule Page/Flow cell
Kinesin technique
Kinesin assays can be very frustrating when things go wrong. There are many steps in the preparation and any one of those steps can cause the assay to not work if improperly executed. Since there are so many steps, the experimenter is left in the dark when things go wrong since it is the final product that we look at and none of the steps in between. In order to prevent inadvertent mistakes from occurring when making flow cells, this page is going to outline how to make them. This flow cell is specific for kinesin assays however, the techniques can apply to preparation of other flow cells for other experiments.
Video
Below is a video describing how to make a flow cell. Continue on to read about how to do it as well.
<html> <object style="height: 344px; width: 425px"><param name="movie" value="http://www.youtube.com/v/wHYCI9OSEcs"><param name="allowFullScreen" value="true"><param name="allowScriptAccess" value="always"><embed src="http://www.youtube.com/v/wHYCI9OSEcs" type="application/x-shockwave-flash" allowfullscreen="true" allowScriptAccess="always" width="425" height="344"></object> </html>
Notes
- Slides should be cleaned. I'm still unsure as to what the best method for cleaning is unfortunately.
- When sticking the tape down on the slide, you should never set it down and then take it off the slide again. When I place the first tape piece on the slide, you will notice that I incorrectly lift it off the slide and place it back down. This leaves tape residue where I pick the tape up from. This is not good for experiments.
- Also when I place the tape down, you will notice that the tape piece is convex in my hand, i.e. it bends towards the table. When placing the tape on the slide, it is best to have the tape in a concave position. It's just easier to place the tape in this position than the other way around.
- So I put the tape pieces rather haphazardly next to each other. This doesn't make for consistent flow cells. What I do now is mark the table with two lines 5 mm apart and line up the tape so that the channel is approximately 5 mm in width. It just makes for a better flow cell.
- I smash the tape down with a box cutter but, this is not necessary with the spaced box cutter apparatus. Just gently push the spaced box cutters on the tape and it should adhere the tape well enough to add the cover slip later.
Flow cell technique
We use slides (#48300-025) and slips (#48366-045) purchased from VWR, pictured below.
When making a flow cell, take out one slide and place it on the counter. You can clean the slide if you wish however, it is not necessary. In the below image, the slide is surrounded by a white box.
Take 2 strips of double stick tape and place them on the slide such that they are approximately 6 mm from each other. The tape should also overhang the slide as shown in the below picture. The reason for doing this is to ensure that any particulates on your gloves do not get any where near the flow cell which is effectively in between the two strips of tape.
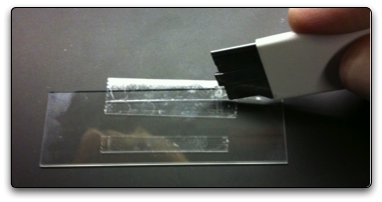
The next step is to use a box cutter to smash the tape onto the slide. The best way to do this is to ensure that the box cutter is flat against the tape and slide and to not use just the tip of the cutter. You want to start from the part of the tape that is closest to the flow cell (in between the two strips of tape) and work your way out to the edge of the slide, smashing the tape onto the slide. Do this for both pieces of tape. Alternatively, if you do not want to use the box cutter as a means to smash the tape to the slide, you can use some high pressure nitrogen gas to get the tape to stick. Just blow the gas on the tape and the force from the gas, if sufficiently forceful, will make the tape adhere to the slide.
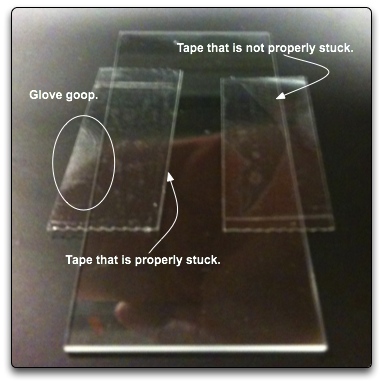
You can see if you have done a good job of making the tape adhere to the slide by looking at how the light reflects off of the tape, seen below. Make sure that the boundary that defines the flow cell is properly stuck to the slide. Otherwise, you will get a leaky flow cell.
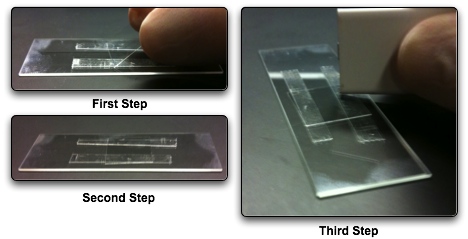
The next step is to take the box cutter and trim the double stick tape. Depending on the size of slip you use, you can take as much or as little off as you want. Typically the slips used in this procedure warrant taking off only a moderate amount of excess tape.
After trimming the excess tape, you will want to put a slip over the tape channel. Start by placing an edge of the the slip on the double stick tape and let it fall on the tape such that it the slip is somewhat squared up with respect to the slide. Once the slip is on the slide, use the box cutter to smash the slip on the tape.
Do not be afraid to apply pressure to the slip in order to make it stick properly to the tape. This is why we try not to take too much of the excess tape off of the slide when trimming. The slip will be supported better with wider strips of tape allowing you to apply decent pressure without it breaking. Once again, you can determine if the slip has properly adhered to the tape by looking at how light is reflected off of the chamber. Below is an image trying to depict this, however, trying to photograph this is not easy. Your eyes and brain are a much better instrument for determining whether or not the slip has been completely smashed on the tape or not.
You can tell somewhat in the above image that there is a darker region on the slip where it has adhered to the tape properly (top tape strip). As opposed to the bottom strip where the slip has yet to be stuck to the tape.
Once you have stuck the slip to the tape completely, you are now able to flow in a motility solution. The approximate volume for a flow cell of this size is about 10 µL. Once the solution is in the flow cell, you must fix the slide. Fixing the slide just means that you cap the two exposed ends of the channel with nail polish. You must do this in order to prevent evaporation from occurring. If you don't, then you will be able to see your sample evaporate under the microscope.
Congratulations on your flow cell. You have made Kiney proud!