User:Alexa Edmier/Notebook/Biology 210 at AU
Embryology Lab
March 22nd, 2014
Introduction:
The purpose of this lab was to determine the effect of alcohol on developing embryos, using zebra-fish as the model organism. The effects of
the alcohol were assessed every few days, over the course of three weeks.
Procedure:
1. Forty zebra-fish were collected and equally distributed between two petri dishes: one with 10 micro-liters of Deer Park Water and the other
with 10 micro-liters of a 1.5% ethanol solution.
2. The eggs were then observed to determine the approximate age, movement, structures, tail length, total length, eye measurements, and
pigmentation (if applicable)
3. The two groups were placed in the back of the lab so they would be undisturbed until the next observation.
4. The solution would be replaced, and the dead organisms removed,once a week. Then, the dependent variables (listed in step two) would be
observed, and differences between the two groups were noted.
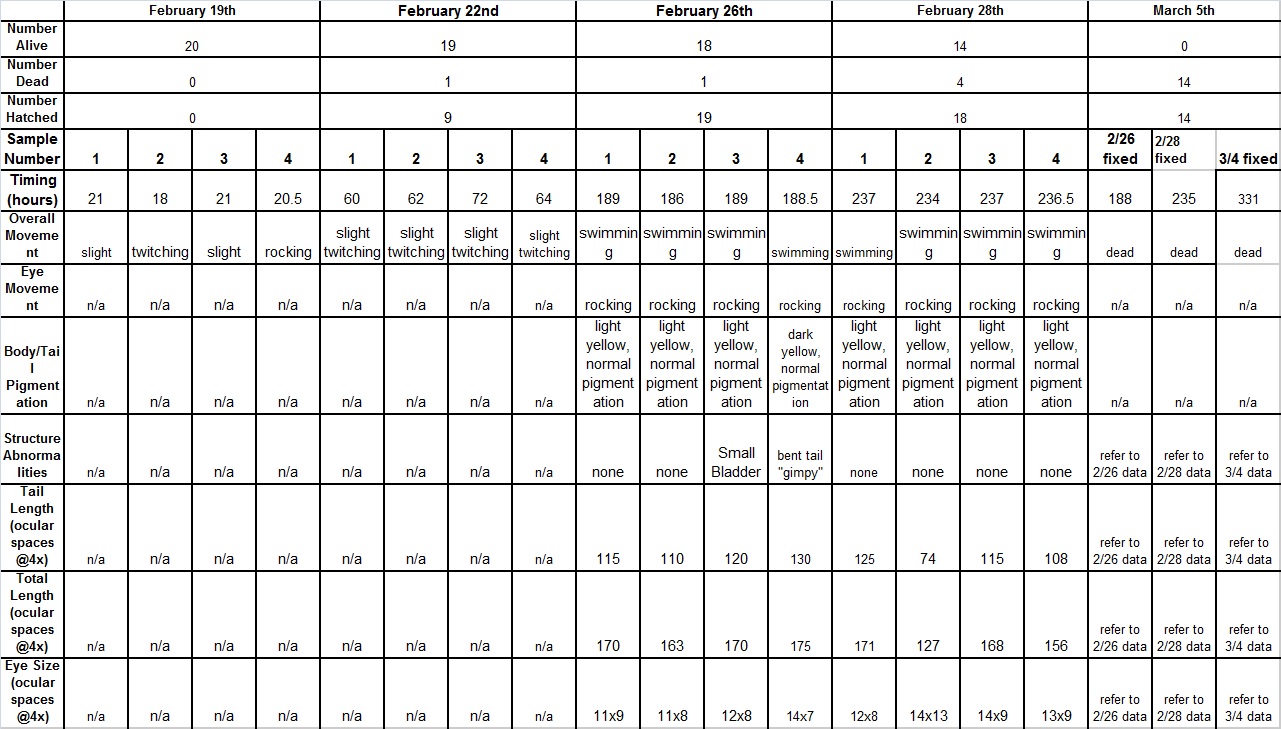
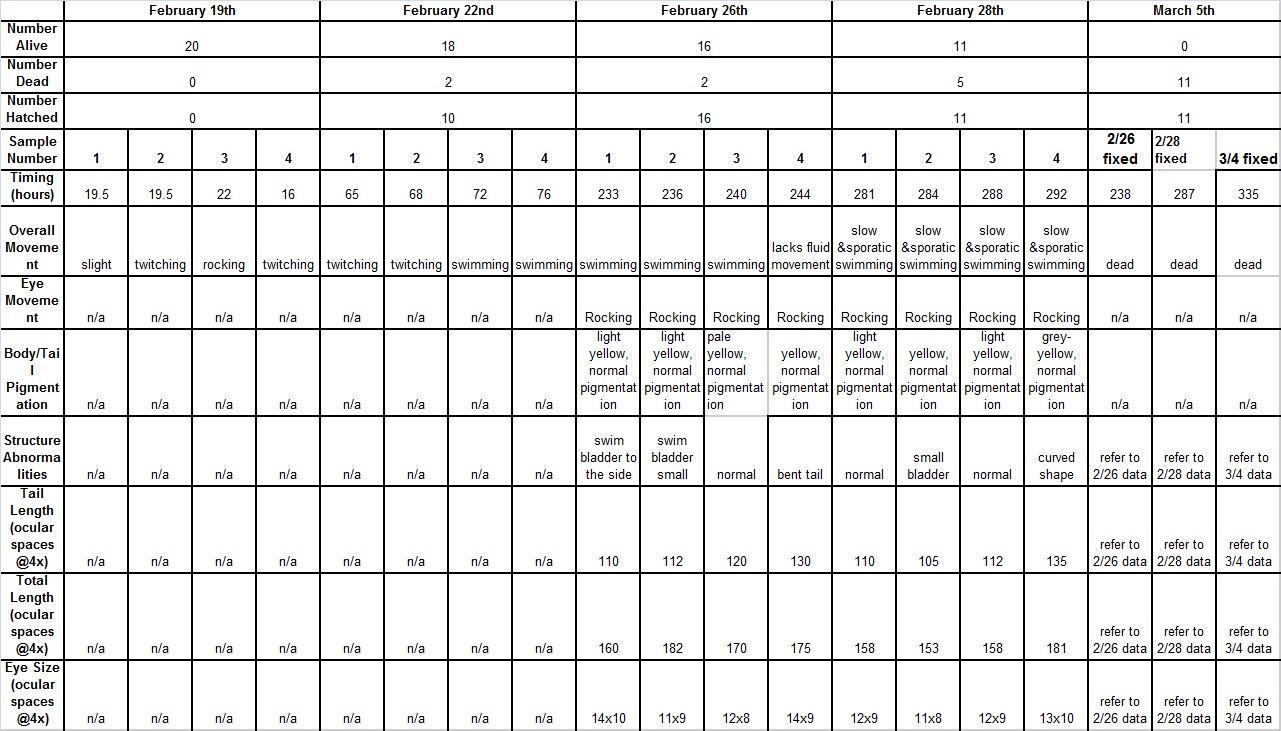
Raw Data:
Conclusions:
Over the course of the experiment, it was noted that the alcohol zebra-fish tended to develop increasing amounts of abnormalities, and had
displayed slower, choppy movement. The fish in the alcohol group also tended to have shorter bodies, with slightly smaller eyes than the fish
in the control group. Additionally, the fish in the alcohol solution died faster than those in the control, and had higher rates of curved
bodies. In conclusion, alcohol has several negative effects on embryonic development, and should be avoided by all organisms.
-AE
PCR Sequence Analysis
March 1st, 2014
Introduction:
The purpose of this lab was to use the sequences found from the PCR reactions to determine the organisms living in our transect.
Procedure:
Reference back to Lab 3: Microbiology and Identifying Bacteria with DNA
Raw Data:
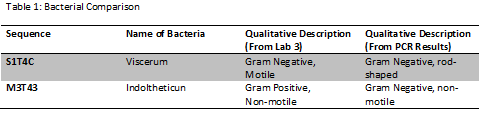
The descriptions of the organisms found in sequences S1T4C and M3T43 are summarized below.
Conclusions:
The sequences found through the PCR reactions were fairly consistent with our observations of organisms back in lab three. Previously we had found both motile and non-motile organisms that were either gram positive or negative, which is what the sequencing found. Overall, we discovered that there are several different organisms living in our transect, but the main ones were both gram-negative organisms.
-AE
Invertebrates
February 25th, 2014
Introduction:
The purpose of this lab is to classify and characterize invertebrates based upon their structure and movement.
Procedure:
1. Prepare slides of three different kinds of worms
2. Carefully observe the worms' movements
3. Collect and characterize the organisms collected in the Berlese funnel
4. Find other vertebrates and niches most likely present in your transect
Raw Data:
Worm Movement
All three type of worms had a slow, contracting type of movement. They appeared to slide across the substance they were placed on, through the contraction of their bodies. This connects back to their body structure because of the link between the worms' circular muscles having the ability to contract and the worms' expansion and contraction during movement.
Invertebrates in Transect
Our soil sample taken in the previous lab did not contain enough leaves, so there were not any organisms for the Berlese funnel to extract. Therefore, we characterized organisms that were brought to the lab as back ups.
Table Link: [1]
Additionally, the dissecting microscopes did not have the ocular ruler, so we were unable to measure the sizes of the different organisms. However, we found that the true bug was the biggest organism while the flea was the smallest. The smaller organisms, such as the fleas and the lice, were more common than the bigger bugs.
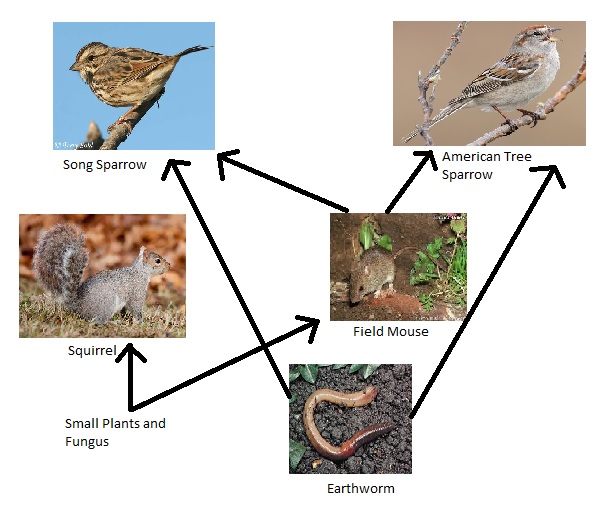
Vertebrates and Our Transect
Table Link: [2]
Food Web:
Conclusion:
I learned how to use a dichotomous key to characterize various different organisms, and learned that soil samples that are mainly comprised of leaves are the most effective when looking to collect organisms. Additionally, we found that there were numerous different kinds of organisms found in each transect, something that I found surprising.
-AE
Plantae and Fungi
February 25th, 2014
Introduction:
The purpose of this lab is to be able to distinguish the difference between plants and fungi, and to characterize each sample into the correct group based on its characteristics.
Procedure:
1. Collect five samples that represent the majority of the plant life in the transect.
-Make sure to collect a variety of samples, from all areas of the transect to ensure diversity
2. Bring the plant samples back to lab and characterize them (based on vascularzation, specialization, and reproduction)
-Look at "veins" to determine if the plant is vascular
-Evidence of seeds allude to the plants' reproduction
-Use of the pamphlets provided in lab allow for the determination of the plants' species
3. Observe and characterize different fungi set up in lab.
-Analyze each fungi sample under the microscope
-Base the characterization on the information in the handouts
4. Set up Berlese Funnel for next lab.
-Take a stand and attach a ring with a funnel
-Position the funnel so it is over a tube (to collect the organisms)
-Carefully put the dirt sample into the funnel, with the leaves at the bottom and soil at the top
Raw Data:
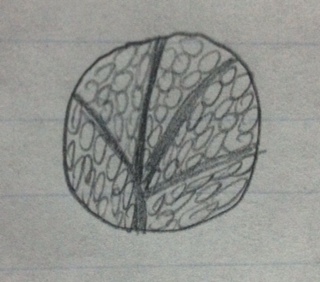
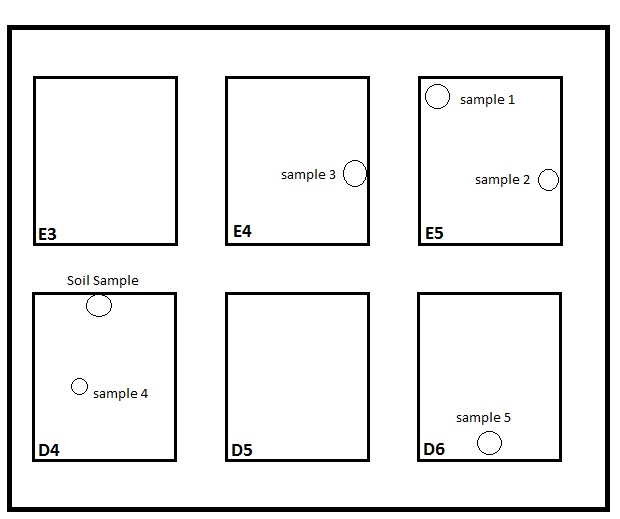
Transect 4 Plant Samples
#1 group and genus
Box E5 Sample 1
Leaf from small bush/vine
Vascular
Rounded
Whirled
Monocot
Plant in its original environment
Plant under dissecting microscope
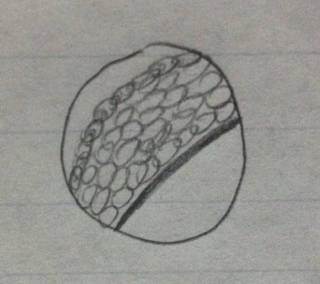
#2 group and genus
Box E5 Sample 2
Bush with long branches
Vascular
Lanced
Compound leaves/leaflets
Monocot
Plant in its original environment
Plant under dissecting microscope
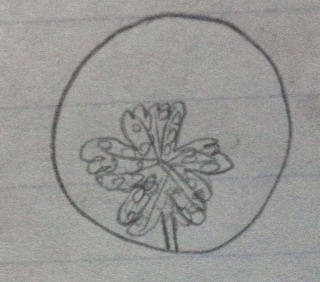
#3 group and genus
Box E4 Sample 3
Small plant
Clover-like
Vascular
Lobed
Whirled
Dicot
Plant in its original environment
Plant under dissecting microscope
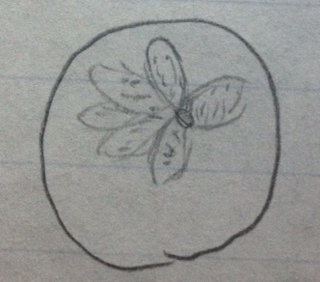
#4 group and genus
Box D4 Sample 4
Small plant/ weed from ground
Vascular
Oval
Compound leaves/leaflet
Dicot
Plant in its original environment
Plant under dissecting microscope
#5 group and genus
Box D6 Sample 5
Seed
Vascular
Is a seed, does not have leaves
Helicopter seed
Monocot
Plant in its original environment
Plant under dissecting microscope
Plant Overview Photo
Transect Map
Fungi
Fungi sporangia are cells within a fungus that contain pores. These cells are detrimental to fungi’s survival because the pores that it holds assist in the reproductive process when released.
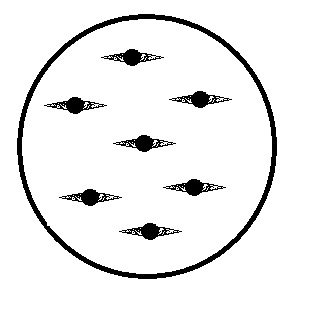
This is easily classified as a fungus belonging to the Zygomycota group because of the evident thick-walled structure (zycospore) that is seen in the center of each spore.
Conclusions:
I found that the majority of plant life in our transect was monocot, with the exception of the few small plants/weeds found. I also found that the majority of plants have visible veins (vascular). However, our transect had very limited plant diversity, because of the cold weather, and I believe that there will be a much greater diversity as the weather gets warmer.
-AE
Microbiology and Identifying Bacteria with DNA
February 16th, 2014
Introduction:
The purpose of this lab was to develop the skills necessary to observe and classify different bacteria.
Procedure:
1. Observe the hay infusion one last time (noting smell and appearance).
2. Analyze the serial dilution petri-dishes to determine the growth in both the nutrient and the nutrient + tet agars, at various different concentrations.
3. Create wet mounds for three of the bacteria cultures (with one being from a tet sample).
4. Observe and characterize the bacteria found in each of these samples
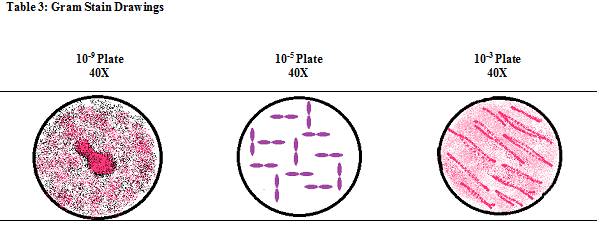
5. Gram-stain each of the samples to determine if they are gram-positive or gram-negative.
-Take a sample of each bacteria and place it on a slide, put on cover slip
-Spray the cover slip with different dyes (thirty seconds for each dye)
-Carefully rinse each slide with distilled water
-Look at the sample under the microscope, if it appears to be pink the bacteria is gram negative, while darker pink-purple appearance
means the sample is gram positive
6. Set up a PCR for one of the cultured samples for next week.
Raw Data:
I do not believe that Archaea species will have grow on the Agar plates because Archaea species are known to live in extreme environments (ex: hot springs and the bottom of the ocean), and the bacteria present in the agar plates has to survive best at room temperature.
Observations:
The growth at the bottom of the jar shrunk significantly
There appears to be less dirt on the bottom
The water is clearer
No film
Less water in the jar
Faint smell
The hay infusion jar will change in appearance and smell over time because the bacteria and organisms will have had more time to reproduce, changing the overall composition of the organisms present in the culture.
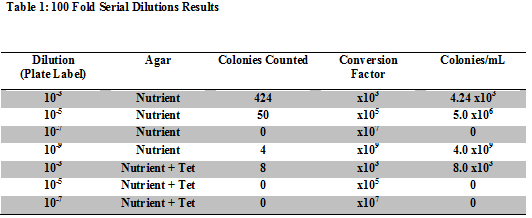
Table 1: 100 Fold Serial Dilutions Results
This shows that there are more colonies present in the plates without the tet, meaning that the bacteria has a more difficult time growing when antibiotics are present.
The presence of the tet limits the bacterial and fungi growth by about 100 times.
There are very few species that are unaffected by the tet, compared to the total number of bacteria that were present in the plates without the tet added to them.
Tetracycline works by binding to the bacteria, inhibiting its ability to create proteins vital to their reproduction (NetDoctor). It is used for a variety of different purposes including: acne, various bacterial diseases, and other infections, provided that the bacteria responsible for the disease/infection has not evolved to become tetracycline-resistant.
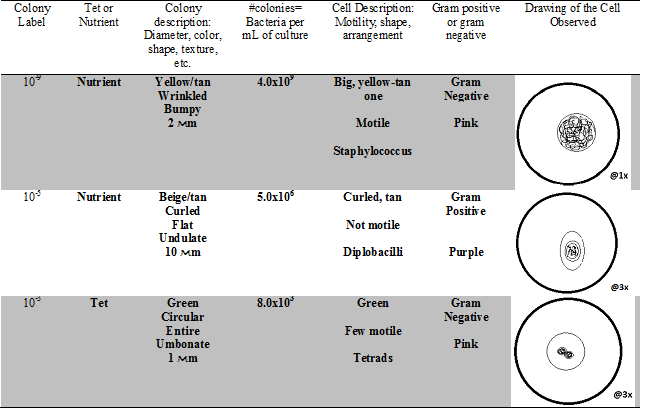
Table 2: Hay Infusion Cell Colony Characteristics
Gram Stain Drawings:
Conclusions:
This lab allowed me to identify different types of bacteria through analyzing the different organisms in the hay infusion culture. Characterization of bacteria can be achieved by observing the size, shape, motility, and staining color of the organism. We also determined that bacteria grows at a much faster rate when tet is not present on the plate, showing that antibiotic severely inhibit the growth of different bacteria.
-AE
Identifying Algae and Protists
February 8th, 2014'
Introduction:
• The purpose of this lab is to distinguish algae from protists through the observation of organisms from different niches in our hay
infusions.
Procedure:
1. Observe and record the characteristics of the hay infusion.
2. Carefully take two samples from the hay infusion (from different areas)
3. Use a dropper to place a drop of liquid from the culture on a slide and place a cover slip on it
4. Characterize the organisms observed on each of the slides
Raw Data:
• Hay infusion Observations-
o Light brown color
o Floating objects on the top
o Clumps of dirt on the bottom
o Film layer on the top
o “fuzz” on the plants
o Potent unpleasant smell
• Sample locations-
o One sample was taken from the top of the culture
o The other sample was collected near the “fuzz” covered plant at the bottom of the culture
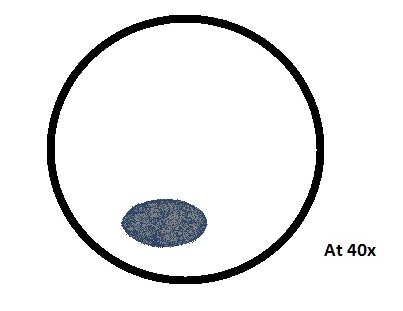
• Organisms from the top sample-
o Volvox
Colored, green, many cells, spherical, colony has more than thirty two cells
100Mm
o Arcella
Colorless, no apparent motion, not spherical, constant shape, pale brown
230Mm
o Stentor
Colored, not green, dark blue
38Mm
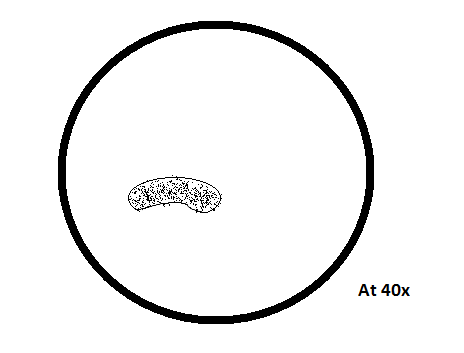
• Organisms from the bottom sample-
o Stentor
Colorless, exhibits other motion, has hair-like structures, body covered with cilia, trumpet-shaped/elongated, attached to substrate
50Mm
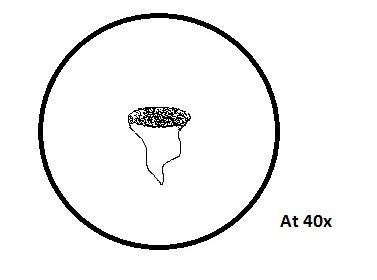
o Vorticella
Colorless, exhibits other motion, has hair-like structures, cilia in specific areas, cell on stalk
75Mm
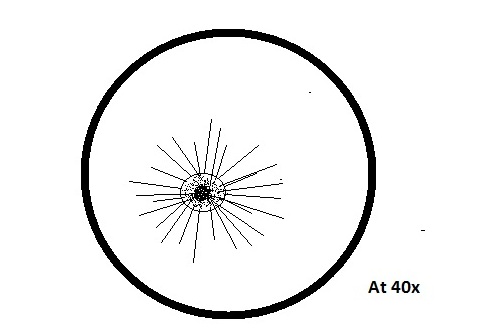
o Actinosphaerium
Colorless, no apparent motion, spherical shaped, radiating spine
87Mm
• Needs of life-
o Actinosphaerium uses its “spikes” to trap smaller protists, to feed on them, for energy. It is multicellular and contains a nucleus,
and uses the fission process for reproduction. It is able to evolve based upon its environment, it was able to survive in our dirty-water
culture even though Actinosphaerium is usually found in clear water. The cells associated with it help with the information transfer to its
nucleus.
• Prediction for Hay Culture- If I were to observe the hay infusion culture for two more months I would expect to see many more organisms, and new growth towards the bottom of the jar. This is because the organisms in the culture would reproduce, and I predict that bacteria in the jar would grow exponentially.
• Possible Selective Pressures- The lack of oxygen (because the jar was covered with a lid) is a major selective pressure that the
organisms in the culture had to face. They were forced to adapt to the lack of oxygen in order to survive, and have most likely found a way to
use water, rather than oxygen, to live.
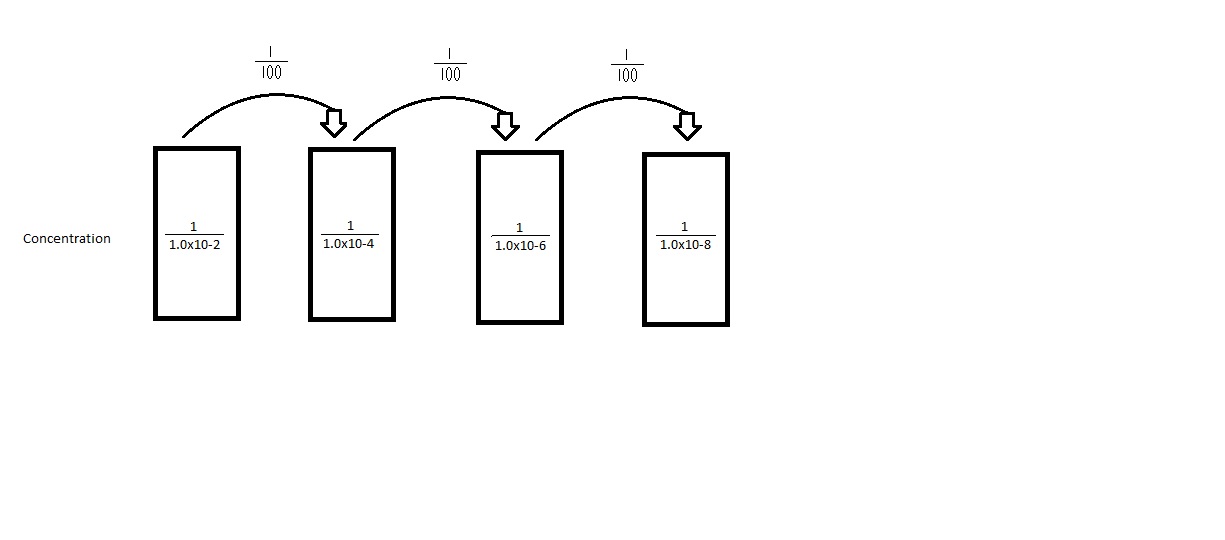
• Serial Dilution Procedure- shown in Figure 1
Conclusions: There were several different organisms in our hay culture, showing that there is a huge variety of organisms in every environment. The organisms present show that while there is a variety, each environment is comprised of organisms that have adapted to it, and have the necessary components to thrive in their environment. I hope to continue to observe these organisms in future labs, to see if they reproduce or grow over time. The origonal purpose of this lab was to be able to identify different protists and algae, something that observing our hay infusion cultures has successfully taught us to do.
-AE
2/6/14, lab 1 notes
Good work! Start working on building a map of your transect to detail your land and where your samples are taken from. We will talk about this more Wednesday. Great job!
AP
Transect Observation
January 15th, 2014
Introduction:
•The lab serves to teach the concept of natural selection through observation of a defined area (transect). The class was divided into five different groups, each of which was then assigned a transect with a unique geographical area. The groups then observed the transect’s topography, biotic, and abiotic factors so they can determine if the transect changes over the course of the semester.
•I predict that the transect will show growth over the course of the semester, because plants are more able to grow in warmer weather.
Procedure:
1.A 20 by 20 foot transect was assigned to each group.
2.The location, topography, biotic and abiotic components of the transect were observed and recorded.
3.A sterile 50mL tube was then used for a soil and vegetation sample
4.10-12 grams of the sample were then measured and mixed with 500Mls of Deerpark water and 0.1 grams of dried milk.
5.The jar was then labeled and left to culture.
Raw Data:
•Transect location- The back right corner of the American University Community Garden
•Topography- mostly sunny (only slightly shaded by trees), not sheltered (windy), good air quality, ability to retain water (in the boxes), and relatively flat.
•Biotic components- leaves, grass, plants/vegetation in box E5, clovers, a bush, roots, and flies. There was also evidence of squirrels, though we did not observe them while we were there.
•Abiotic components- wood, rocks, mulch, soil, and dead grass.
Conclusions:
•Our transect did not show much growth, there was only vegetation in one of the four boxes observed, most likely due to the cold weather. The sample taken will be observed closer next week in lab, so that we may better understand the many organisms in our transect. Natural selection was not observed this week, most likely because of the cold weather, but I believe that it will be observed when the weather warms up.
-AE
January 22nd,2014 Successfully entered text -AE