User:Abbey Butner/Notebook/Biology 210 at AU
2/23/2014 Lab 5 The purpose of this lab was to understand the different characteristics of invertebrates, and to learn how simple systems evolved into more complex systems. The first portion of this lab was to observe three different species of worms. The first worm we observed was the platyhelmenthes, more commonly known as the flat worm. It is an acoelomate, and looks like a mini slug. It was gliding along the bottom of the dish, and it didn't seem to scrunch up when it moved. It was black, with a grey opening in the middle of it, which is used for digestion and reproduction. The next worm was a a pseudo coelomate, from the nematoda family. It was only able to be seen under the microscope, and it was very skinny and light colored. Its body was contracting to move. The final worm was an annelida, which is part of the coelomate family. This is a common earth worm. It had very complex movements, and it could move multiple parts of its body at once. Pictures of all of the worms are below, with labels.
Flat worm: platyhelmenthes
Nematoda: pseudo coelomate
Earthworm: annelida
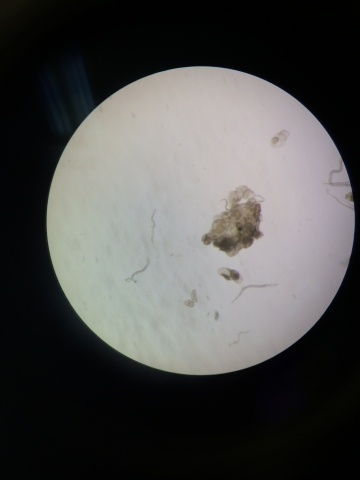
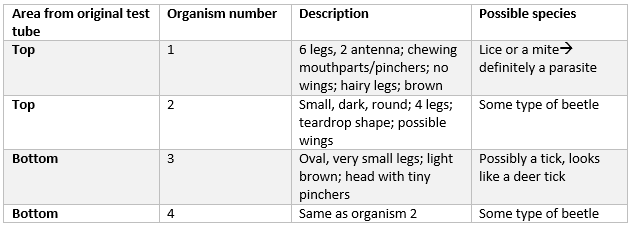
The next portion of the lab was observing the invertebrates that fell into the test tube from the berlese funnel. The test tube's contents were separated into two different test tubes-- one with solution and organisms from the top of the test tube, and one with solution from the bottom of the test tube. The table below shows the different characteristics of the organisms found in the test tube.
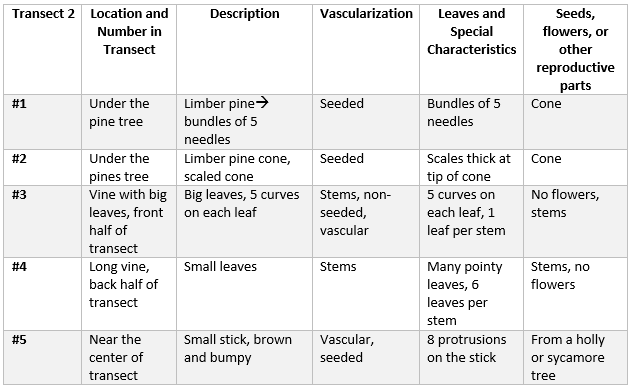
2/23/2014 Lab 4 The purpose of this lab was to understand the different characteristics of plants, and to also understand the various functions of fungi. The first part of this lab was to collect five plant samples from the transect, along with a leaf litter samples. For the leaf litter sample, the leaves and plant matter above the ground, and the first crumbly layer of soil were collected in a plastic bag. Then, five different types of plants/plant leaves were collected from the transect. In the table below, the five plants samples are listed along with their different characteristics.
Next, a sample of moss (bryophyte)and a lily (angiosperm) were compared. Bryophytes do not have vascular tissue to carry water and nutrients around the plant, so they use rhizoids instead. Rhizoids are small, hair-like projections that anchor the plant so that it can grow on the ground. Bryophytes take water from their surfaces and distribute it via diffusion. The rhizoids on the moss are around 8.5 cm long. However, the lily's stem was 24 inches long. Because the lily is an angiosperm, is has a stem which contains the xylem and phloem layers. These layers transport nutrients up and down the flower, and act as a support tissue. Since the lily is more complex than the moss, it has bigger structures. During this portion of the lab, we looked at the moss and lily under the microscopes. It was interesting to see the differences in vascularization in person that close up. There were no seeds in any of the plant samples from our transect, however, in the spring there will be seeds in the pine cones from the pine tree. And the vines will have seeds in the ground too, but since it is winter there was no evidence of any seeds. When observing the fungi under the microscope, the sporangia can be seen. These are protrusions from the main body of the fungus that is involved in the asexual reproduction of spores. This is important, because without these sporangia, the fungus could not reproduce. The next part of the lab was setting up the Berlese Funnel to collect invertebrates. The leaf litter was put into a funnel with a mesh net over the opening at the bottom to prevent dirt and other debris from getting into the test tube under the funnel. The test tube contained 25mL of water and 25mL of alcohol. A light fixture was set up above the funnel, and turned on. Throughout the next week, the invertebrates in the leaf litter will try to escape from the heat if the lamp, and move further into the leaf litter, eventually falling into the alcohol and water mixture. The alcohol will kill them, so that next week they can be observed under the dissecting microscope.
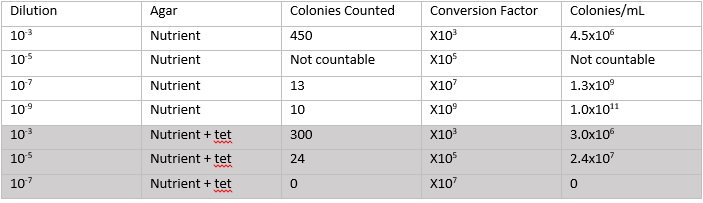
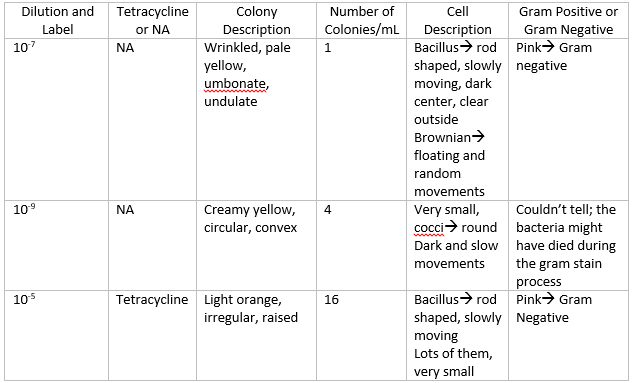
2/17/2014 Lab 3 The purpose of this lab was to understand the different characteristics of bacteria, including antibiotic resistance. The first part of this lab was observing the bacteria that grew over the week on the agar plates. I made a table noting the amount of bacteria that grew on each of the agar plates, which is shown below.
The plates without the antibiotic had a lot of different bacteria growing on them, and the plates with the antibiotic only had one species of bacteria. The species was orange, and grew in round colonies. The orange bacteria grew on both sets of plates, which means that it can grow with just plain nutrient, and even with antibiotics, so it is antibiotic resistant. The tetracycline killed all of the other bacteria by inhibiting the enzymes that are responsible for vital processes within each bacterium. Tetracycline works by binding to the 30s ribosome of the bacterium, preventing the attachment of the aminoacyl tRNA to the RNA ribosomal complex. This means that proteins cannot be synthesized, and it also causes the bacterium to leak nucleotides. Instead of killing the bacterium, it inhibits it, causing the bacterium to not be able to replicate. Bacteria like E. Coli, haemophilus influenzae, mycobacterium (tuberculosis), and pseudomonas aeruginosa are particularly sensitive to tetracycline. (Source: Klajn, Rafal. "Tetracycline - Antimicrobial Properties." Tetracycline - Antimicrobial Properties. Institute of Organic Chemistry, PAN, n.d. Web. 16 Feb. 2014.)
My lab partners and I then observed three different colonies of bacteria from three different plates. Two colonies were from a regular nutrient agar plate, and the other colony was from a nutrient and tetracycline plate. The table below shows the colony descriptions along with the cell descriptions.
During the process of observing the bacteria, we gram stained each individual bacteria colony. To do a gram stain, you make a wet mount of the colony on a slide, then pass it through a flame three times with the bacteria smear side up. The rinse the stain with water. Cover the smear with Gram's iodine mordant for 1 minute. Rinse it gently with water. Then decolorize the smear by rinsing it with 95% alcohol for 10-20 minutes. Rise it with water. Cover the smear with safarin stain for 20-30 seconds, and then rinse it gently. Blot the excess water with a paper towel and air dry.
The gram stain worked for two out of the three slides. The one it didn't work for was the 10^-9 nutrient agar plate. The bacteria was probably accidentally washed off during one of the rinsing processes. To observe the other gram stains, the slide was put into focus on the 40x objective, and then observed by the 100x oil objective. The gram results are in the colony description table.
Lastly, a PCR reaction was prepared for the next lab. A PCR product was prepared for each of the colonies observed on the slides.
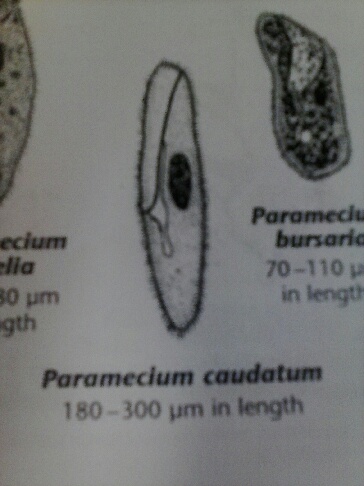
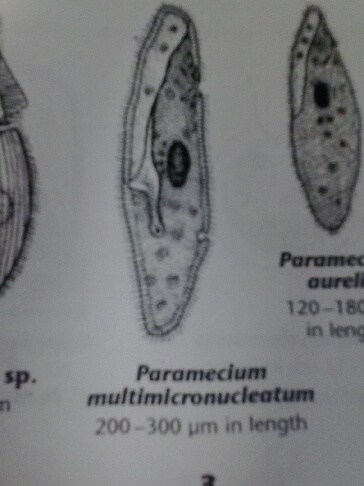
2/9/2014 Lab 2 The purpose of this lab was to determine different types of bacteria from a sample in the lab and a sample from the hay infusion. To do this, a dichotomous key was used, along with a diagram showing different bacteria colony morphologies. In the lab sample, three different organisms were determined by using a microscope. The first organism was a paramecium, which was around 300um, which means it was either part of the multimicronucleatum species or the caudatum species. Below are two pictures from Ward's Free-Living Protozoa manual.
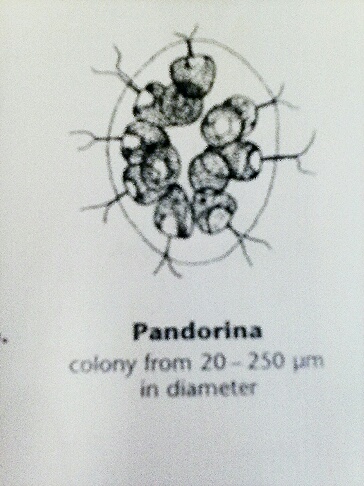
Another organism found in the lab sample was a pandorina, which was around 25um. This organism was swirling around on the slide, and was round and green. A picture below, also from Ward's Free-Living Protozoa manual shows what a pandorina looks like under a higher objective. Since this organism was moving, it could only been seen under the 40x objective, which did not show much of its detail.
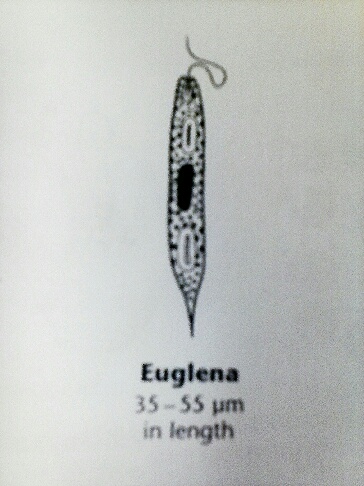
The last organism observed in the lab sample was an euglena, which was around 40um. This organism was long and skinny, and moved in a spinning/twitching motion. Again, it was observed under the 40x objective, so the picture below from Ward's Free-Living Protozoa manual shows it in better detail.
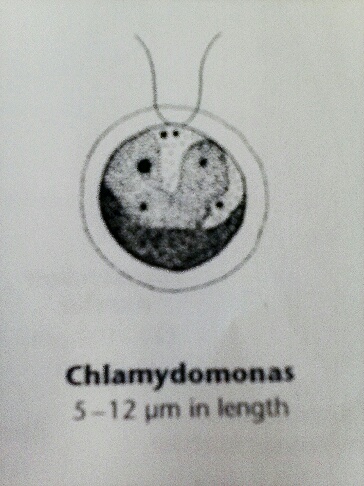
The next part of this lab was to observe the hay infusion culture made at the end of Lab 1. The top of the infusion had a thin white layer of mold, and the liquid had a horrible smell. The leaves and small sticks had settled near the bottom of the infusion, and the dirt was at the very bottom of the jar. My lab partners and I found two organisms from two different niches in the hay infusion, and then characterized the organisms in these niches. The first niche was by some plant matter. One organism observed in this niche was a chlamydomonas, which was around 5um. The organism was slightly green, which was not surprising since it was near the plant matter. Another organism that was observed in this niche was an euglena. Slightly bigger than the chlamydomonas, it was about 30um. Since both of these organisms were so tiny, it was difficult to see all of their characteristics under the microscope, so there are pictures from Ward's Free-Living Protozoa manual. The picture below is of the chlamydomonas, and a picture of the euglena is shown above.
The next niche that was observed was near the dirt at the bottom of the infusion. Another chlamydomonas was found in this niche as well, along with a paramecium. The chlamydomonas in this niche was particularly active, and its movement resembled that of a centipede's. It seemed to inch along very slowly, but it was definitely moving. This chlamydomonas was also similar in size to the chlamydomonas found in the plant material niche. The paramecium was scooting around through the infusion pretty quickly, which made it hard to see its features in detail. There are pictures of both the paramecium and chlamydomonas in this lab entry. The paramecium is a good example of an organism that meets all of the criteria for being alive. It reproduces through asexual reproduction; it uses its many cilia to capture prey; there are many species of paramecium, which shows how it has adapted to different environments; it has a nucleus and other organelles; finally, it can adjust to environmental conditions by moving from one niche to another depending on environmental stimuli.
If the hay infusion culture had been observed for another two months, the plant material would probably have decomposed into dirt-like organic material, meaning the organisms that live in the plant material niche would be dead. However, there would probably some adaptive radiation by the organisms in the dirt niche since their niche would have expanded. The mold at the top of the fusion would have grown even thicker, and the smell would most likely be extremely horrible because of all the decomposition occurring.
Next in the lab, a serial dilution of the hay infusion was made. There were four nutrient agar and three agar plus tetracycline plates made. The plain agar nutrient plates had the concentrations of 10^-3, 10^-5, 10^-7, and 10^-9. The tetracycline plates had concentrations of 10^-3, 10^-5, and 10^-7. Both sets of plates were left to incubate at room temperature over the next week.
1/31/2014 Lab 1
The purpose of this lab was to define different niches at American University. A niche is a specific set of requirements for living that organisms share. During this lab, my lab partners and I studied a 20 by 20 foot transect near the Seminary on Massachusetts Avenue. The procedure for studying the transect is listed below.
Procedure:
1) Define the 20 by 20 foot area to study. Mark this area with red flags.
2) Describe the general characteristics of the transect, like location, topography, etc.
3) Make a list of the abiotic (nonliving) and biotic (living) components in the transect.
4) Collect a sample of some of the components using a sterile 50ml conical tube. Be sure to include soil and vegetation.
5) In the lab, make a hay infusion culture by weighing 10 to 12 grams of the soil/ground sample and place in a plastic jar with 500 mls of deerpark water.
6) Add .1 grams of dried milk and mix gently.
7) Take the top off of the jar, label it well, and place it in the lab.
Below are some pictures from the transect.
In our transect, the five most noticeable abiotic components were a tennis ball, bench, a dog poop bag, aluminum foil, and dead branches. The four most noticeable biotic features were the full-grown trees, vines on the trees, sprouting trees, and ivy ground-cover.
The total weight of the sample we collected from our transect was 11.65 grams.



Good start. Needs some more detail on what you did rather than just writing out the directions from the manual. Address all red text from protocol and include conclusions etc. Include the volvocine line observations. For more instructions see TA notebook. SK
1/23/2014 Was able to type stuff into my notebook! AB