Tissue Engineered Liver by Brittany Shepler, Thomas McCarthy
Background
Every year within the United States, upwards of 36,000 people die from chronic liver diseases or their complications [8], making liver failure the 12th most frequent cause of death. Of the 36,000 patients, only 17,000 are selected and approved for transplants. While there has been an increased number of transplants over the last couple decades, typically 1,500 patients still die every year while awaiting a liver transplant [7], and is unlikely to improve meaning liver transplantation alone will never meet the demand. Liver disease is roughly grouped into three categories, chronic liver disease, acute liver failure, and chronic liver failure. These groups represent, metabolic dysfunction meaning unrelated to trauma or tissue scarring (i.e. genetic disease), direct injury and loss of functional cells (hepatocytes), and widespread tissues damage, scaring/remodeling or cirrhosis, respectively [1]. Treatment of liver disease and failure can be accomplished through organ and, with limited successes, cell transplantation. Unlike other organs, like kidneys and hearts, extracorporeal devices are still only in clinical testing due to the unique and hard to replicate structure of the liver. While, engineered tissues are being developed from both reengineered bio-derived tissues and synthetic materials, no one has developed are fully function replacement. Under current technology, the only option for liver failure is an organ transplant from a human donner.
The Liver
The liver makes up 2 to 5% of the human body weight making the organ the largest in the body, and performs more than 500 complex array of functions in metabolic, synthetic, immunologic, and detoxification processes. Remarkably, the organ has a large capacity for regeneration; in which, only one-third of the total cells need to remain uninjured in an acute failure for potential full restoration [16]. This allows for partial transplants, where only right hepatic lope is need from the donner. However, liver regeneration is not fully understood and difficult to fully predict. The organ’s structure is a multicellular network in which the main cells, hepatocytes, are arranged in a sandwiched by extracellular matrix (EMC) in a sinusoidal fashion [1]. These hepatocytes are supported by a range of other cell types like, endothelial cells, Kuppfer cells, biliary dutcal cells, and stellate cells. Hepatocytes have high metabolic rates and as such are exposed to high gradients of nutrients, hormones, and growth factors through a complex and high visualized network. This has made cell culture of hepatocytes difficult as they rapidly lose liver-specific functions and the ability to replicate (especial at high rates), due to their unique environment.
Chronic Liver Disease
This category largely makes up liver-based metabolic disorders coming from defects in enzymes and transport proteins. Some examples include, Crigler-Najjar syndrome, phenylketonuria, and oxalosis [1]. This category is marked uniquely by the lack of tissue scarring architecture and is the most likely to show successes in cell (hepatocyte) transfer therapies.
Acute Liver Injury
Injuries that occur to the liver in which rapid deterioration of cell function happened in less than 26 weeks are classified as acute [1]. This can be tested for as when hepatocytes necrosis, increase levels of liver-specific enzymes like alanine transaminase (ALT) and aspartate transaminase (AST) increase. Depending on the type and severity damage, acute failure can lead to mortality but at the same time, due to the regenerative nature of the liver, complications can resolve spontaneously. This is clinically hard to predict and better models are needed to prevent unnecessary transplants.
Chronic liver Failure — Cirrhosis
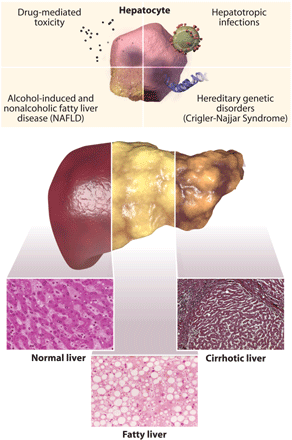
Cirrhosis is the hardening of the liver and development of scar tissue. This may be caused by a range of stimuli, including alcohol abuse, illness, and failure in other areas of the body [10]. While scar tissue in itself is not inherently dangerous, its ability to prevent the flow of fluids (i.e. blood) can prove problematic. This would prove particularly true since the liver functions as a filtration system, metabolism regulator, and protein generator. Complications of cirrhosis include excessive bleeding and hemorrhaging, namely around the portal vein that connects the digestive tract and the liver, hepatic encephalopathy (toxin poisoning), and organ failure (i.e. kidney, pancreas) [10]. While most of these complications may be abstained, a liver transplant becomes necessary once treatment becomes ineffective.
Fatty Liver Disease
Fatty liver disease occurs when there is an unnatural amount of fat present within the liver. This usually occurs when the organ has trouble breaking down fat and thus causes an accumulation [10]. There are two types of fatty liver disease: alcoholic and nonalcoholic. Both forms may ultimately lead to cirrhosis and end stage liver disease.
Alcoholic fatty liver disease occurs when an individual undergoes alcohol poisoning on a reoccurring basis. As a result, the vast majority of alcohol abusers develop fatty livers [10]. Not all of them, though, are aware of the disease or only have minor development.
Nonalcoholic fatty liver disease is one of the leading causes of chronic liver disease within the United States [10]. However, it has no determined cause. While it has been found that people who divulge in poor diets are more likely to develop this disease, there is no confirmation that this is the source of the problem
Hepatitis
Hepatitis is the inflammation of the liver commonly stimulated by a virus. While there are numerous strains present in the human body, five prove to be the most prevalent. These are hepatitis strains A through E, with strains A through C being the most common [10]. While all of these strains can lead to liver failure, hepatitis B and C are the most common lines to develop beyond acute inflammation, cause chronic illness, and spread between individuals [10]. If hepatitis develops into a chronic condition without early treatment, cirrhosis or liver cancer may develop.
Hemochromatosis
Hemochromatosis Hemochromatosis is a primarily hereditary disease where too much iron is absorbed and stored in the body [10]. While this disease does not solely effect the liver, its impact can lead to cirrhosis. This is due to excessive filtering of the harsh mineral as well as potential buildup of the ion within the liver.
History
The development of usable, engineered liver tissue is still relatively new. The use of liver transplants and artificial systems however, has been taking place since the early 1960s [11], first transplant by Dr. Thomas Starz. The first successful human liver transplant was performed in 1967, and in 1983 liver transplant became recognized as the leading medical therapy for end stage liver disease [11]. Liver and cardiovascular tissue engineering began in the 1990s, followed quickly by the development of a tissue engineering society [15]. From there, tissue engineering has become a developing field, resulting in the first developments of liver tissue in the 2010s. Fully developed livers are predicted to be developed sometime in the next decade [2].
Engineered Livers
Liver Transplants
To date liver orthotropic transplants remain the only treatment for liver failure. The Procedure can take up 4 to 18 hours with multiple surgeons costing $250,000 [17]. The process involves multiple reviews by medical teams to evaluate the need for the transplant and medical test for a correctly match organ and making sure the patient is medically heathy enough for the surgery. Due to the regenerative nature of the organ only a section of the organ is need for transplant. While most transplants are from diseased donors, a liver is capable of being taken from a living donor. After a year 80 to 85% of all organ transplants are still functioning [17].
Cell Implantation at the Liver
Studies have been done where isolated hepatocytes are directly implanted into the portal vein of the liver. Encouraging results followed, namely for patients who suffer from minor acute or functional disease [13]. However, this therapy proved to be inefficient for one primary reason: the number of cells that may be transplanted is limited [13]. More than half of the material found within a fully developed liver consists of excess fat or plaque that renders the tissue unusable. Furthermore, injecting a collection of foreign cells could trigger and unwanted immune response, essentially leaving the patient in similar or worse condition than when the implant was first received.
Extracorporeal Devices
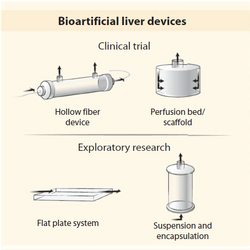
These devices are aimed not be a permanent solution to organ failure, but as a temporary support until a transplant can be done. These devices are done in the same vain as hemodialysis, but as hepatocyte function is challenging to recreate, most current research on such devices that use the cells in what’s called a bioartificial liver (BAL) [1]. As human derived hepatocytes are hard to acquire BALs often use xenogeneic sources. The most common design and currently in cincal trail is hollow fiber models where the liver cells are culture on the fibers. Other models use suspensions of cells often as encapsulated spheroids [18]. The set back with the BALS is directly related to the challenge in culturing hepatocytes in retaining their phenotype for prolonged periods of time. Additional the devices also have the challenge of dealing with shear stress and oxygen tension.
Challenges
Hepatocyte Culturing
Hepatocytes are difficult to harvest. That is to say, when a sample of human hepatocytes is collected and placed into a culture, they tend to lose their liver-specific functionality [5]. As a result, these cells lose their ability to metabolize drugs and other media [1]. If researchers were able to stimulate proliferation of these cells, it would thus prove pointless since the cells would no longer be able to perform the filtration and regulation processes they normal do.
Hepatocytes Development
While there are proven ways to form hepatocytes using materials such as growth factors and cytokines, researchers have yet to fully illustrate a signaling pathway that encourages mesenchymal stem cells to differentiate into hepatocytes [6]. The signaling pathway is important because it helps stimulate cell functions like tumourigenesis, apoptosis, and cell-fate determination. In addition, the signaling pathway acts as a resource of information about cell-cell and cell-matrix interactions and regulation. Discovering what mechanisms trigger these responses could help eliminate formation of tumors and unwarranted cell death in newly grown liver tissue. Without the ability to fully demonstrate the hepatocyte proliferation and differentiation process, however, the effective use of mesenchymal stem cells for therapeutic liver disease treatments is limited [6].
Cell Implantation at the Liver
Studies have been done where isolated hepatocytes are directly implanted into the portal vein of the liver. Encouraging results followed, namely for patients who suffer from acute or functional disease [13]. However, this therapy proved to be inefficient for one primary reason: the number of cells that may be transplanted is limited [13]. More than half of the material found within a fully developed liver consists of excess fat or plaque that renders the tissue unusable. Furthermore, injecting a collection of foreign cells could trigger and unwanted immune response, essentially leaving the patient in similar or worse condition than when the implant was first received.
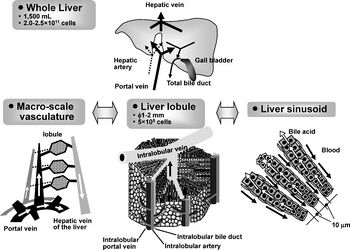
Vascularization
As of the moment, sheets of liver tissue can be produced. An entire liver cannot, however, due to the complex vascularity system present within the tissue. Since the liver is the primarily location for metabolism, blood vessels are spread out in a fashion that ensures every hepatocyte may be used for metabolic processes [2]. This wide spread system is what makes engineering tissues difficult.
While is it not crucial that engineered tissues perfectly mimic that of an organic liver, it is imperative that the implanted tissue perform the same amount and range of the tasks as the previously healthy tissue [2]. The most important task is to allow the flow of blood through the organ. While techniques that involve engineering 2D layers of tissue and stacking them have become present, forming uniform and well spread channels between these layers to allow blood circulation still continues to be a problem.
Recent Studies
Culturing Human Liver Sinusoid
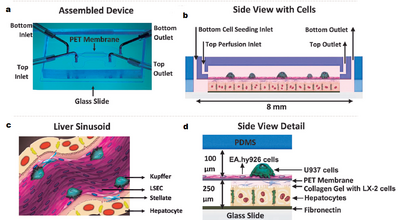
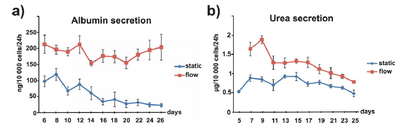
The challenge in culturing hepatocytes is in their unique environment, and efforts to replicate this environment has proven to be challenging. With this means that hepatocytes will lose their phenotypic response and function rapidly. This is a fundamental challenge in the development of BALs. Researchers at Massachusetts General Hospital, have been working a microfluidic 3D device that mimic the sinusoid and are able to maintain primary cells at hepatocyte function for over four weeks [19]. The device used a 3D gel layout based around PDMS-PET and liver associated cells. After a period of four weeks the cells produced high levels of albumin and urea indicating liver function, figure 4.
Liver Preservation
As currently the only solution for complete liver failure is a donor transplant much effort has gone into the preservation of organs from recently diseased donors. The organ suffers from warm ischemia like most organs but heighten due to high metabolic activity. Currently, the solution is static cold storage (SCS) or placing the organ on ice. Two solutions are currently being reasearch into how to prolong the storage of livers so less will become damaged in transport to the patient. These potential solutions are machine perfusion and supercooling.
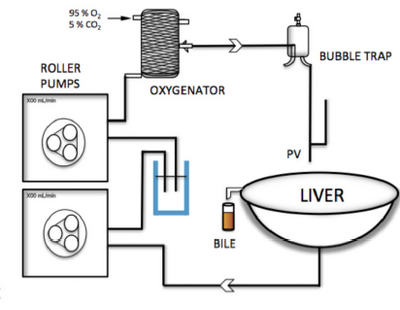
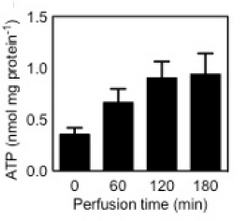
Machine perfusion, Figure 8., is the process of pumping oxygenated plasma into the liver to slow down warm ischemia and it has also shown to regain function of the organ recently damage from ischemia [20]. As shown in figure 6, after an hour of simulated warm ischemia machine perfusion was able to regenerate ATP activity 2.8 fold.
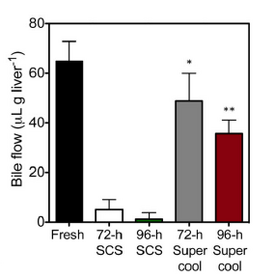
Supercooling shows the greatest advancement in prolonging the storage of the organ reaching a 68% recovery of rat liver after 96 hours at -6°C. Degradation of tissues will be slower at lower temperatures, however, below the freezing point of water, cells will begin to rupture. To combate this testing has been done on rat livers where the organs where injected with cryoprotectants to stop the organ from freezing at below zero temperatures. The cryoprotents used where 3-O-methyl glucose (3-OMG) and polyethylene glycol (PEG). These together lower the freezing point and prevent ice crystal formation. When implanted back into rats can compared to SCS method at similar times, bile product was significantly higher for supercool livers figure (Figure 7).
Extrahepatic Injection
Since prior studies have shown complications that accompany direct implantation of hepatocytes to the liver, researchers have started investigating injecting them into extrahepatic areas [5]. Key areas of investigation are beneath the kidney and within the subcutaneous space. These areas were chosen because the kidney proved to be a prosperous place for islet implantation and the subcutaneous space due to its easy access.
In a recent study, a collection of human hepatocytes were implanted into mice, either beneath the kidney or in the subcutaneous space [5]. It was found that with the proper support of a membrane protein (i.e. laminin, collagen) enriched extracellular matrix, hepatocytes were persistent for a minimum of 20 weeks. However, hepatocytes implanted away from the liver without the support of an extracellular matrix would de-differentiate after approximately 3 weeks [5]. It was only when the hepatocytes had been suspended in an extracellular matrix that they would continue to differentiate as liver cells.
The tissues underwent either a two-third hepatectomy or intragastric injection to determine whether or not the injected hepatocytes were proliferating. These tests concluded that not only were the cells growing, but they also existed in quantities relatively close to what was organically found within the mouse [5].
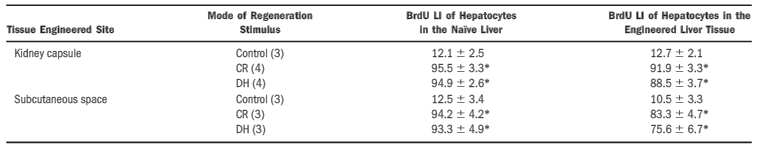
It should also be noted that while both locations manifested a great deal of liver tissue, the location beneath the kidney proved to be more effective. Furthermore, a significant amount of the hepatocytes that were injected within the subcutaneous space had died within the first 2 weeks of study [5]. Thus, further research will need to be done to determine whether or not tissues may be produced in the subcutaneous space.
Decellularization of Porcine Livers
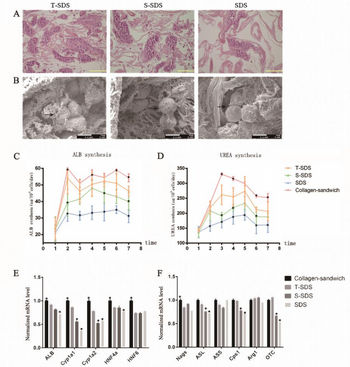
There is research currently being performed to standardize a method of decullarization of porcine livers [4]. Decellurization occurs when an organ or tissue is removed from a donor and the cells are killed, leaving only the extracellular matrix behind to act as a scaffold. This could prove useful because it removes the threat of an unwarranted immune response while leaving behind vascular patterns and biological cues that lead to cell differentiation into hepatocytes [4].
The decellularized livers act as biological scaffolds, ultimately providing a welcoming environment for stem cell adhesion and differentiation [4]. They could then be used to develop autologous liver graphs, allowing for biologically identical tissues to be implanted into an individual. While this has promise of further developing into a commonly practice protocol, it would still act as a temporary therapy for persons awaiting liver transplant.
The latest study has shown a procedure that allows for 70% retention of collagen and glycosaminoglycans (GAGs) when using a sodium dodecyl sulfate (namely T-SDS) solution for decellularization [4]. Furthermore, upon injecting hepatocytes collected from rats into the liver graph of the decellularized extracellular matrix, it was found that urea and albumin production was significantly increased from previous studies. The expression of three out of the five liver-specific genes was also present [4]. This demonstrates that the liver had functionality. Overall, this procedure has promise for in vitro study, but it remains an impermanent solution to the deficiency of livers available for transplant.
Future Research
Numerous researchers and companies are actively working to try and develop a 3D printed liver that would be available for transplantation. Model livers are currently being printed for operation practices and liver tissue is available in single sheets. This is done by alternating layers of hydrogels and cells, then solidifying the printed object via temperature-controlled properties [2]. But while 3D printing has made extraordinary advances, it has not been able to successfully graph the vascularity of multilayered liver tissue. Once this roadblock is resolved, livers should be readily available at the click of a button.
References
[1] S. N. Bhatia, G. H. Underhill, K. S. Zaret, I. J. Fox. “Cell and tissue engineering for liver disease”. Science Translational Medicine. 6, 245, p. 245sr2 (2014).
[2] Y. Sakai, H. Huang, S. Hanada, T. Niino. “Toward engineering of vascularized three-dimensional liver tissue equivalents possessing a clinically significant mass”. Biochemical Engineering Journal. 48, 3, p. 348-361 (2010).
[3] L. G. Griffith, G. Naughton. “Tissue Engineering--Current Challenges and Expanding Opportunities”. Science. 295, p. 1009-1014 (2002).
[4] Q. Wu, J. Bao, Y. Zhou, Y. Wang, Z. Du, Y. Shi, L. Li, H. Bu. “Optimizing perfusion-decellularization methods of porcine livers for clinical-scale whole organ bioengineering”. BioMed Research International. (2015).
[5] K. Ohashi, J. M. Waugh, M. D. Dake, T. Yokoyama, H. Kuge, Y. Nakajima, M. Yamanouchi, H. Naka, A. Yoshioka, M. A. Kay. “Liver Tissue Engineering at Extrahepatic Sites in Miceas a Potential New Therapy for Genetic Liver Diseases”. Hepatology. 41, 1, p. 132-140 (2005).
[6] J. S. Yi, X. S. Su, J. F. Stoltz, N. de Isa, L. Zang. “Signalling pathways involved in the process of mesenchymal stem cellsdifferentiating into hepatocytes”. Cell Proliferation. 48, 2, p. 157-165 (2015).
[7] American Liver Foundation. “More About Organ Donation”. http://www.liverfoundation.org/patients/organdonor/about/
[8] Center for Disease Control and Prevention. “Chronic Liver Disease and Cirrhosis”. http://www.cdc.gov/nchs/fastats/liver-disease.htm
[9] U.S. Department of Health and Human Services. “The Need is Real: Data”. http://www.organdonor.gov/about/data.html
[10] Mayo Clinic. “Liver problems”. http://www.mayoclinic.org/diseases-conditions/liver-problems/multimedia/liver-problems/img-20009008
[11] C. Manzarbeitia. “History of the Procedure”. MedScape. (2014).
[12] Nature Biotechnology. “Tissue Engineering”. Nature Biotechnology. 18, p. IT56-IT 58 (2000).
[13] S. C. Strom, P. Bruzzone, H. Cai, E. Ellis, T. Lehmann, K. Mitamura, T. Miki. “Hepatocyte Transplantation: Clinical Experience and Potential for Future Use”. Cell Transplantation. 15, 1, p. S105-110 (2006).
[14] F. G. Court, S. A. Wemyss-Holden, A. R. Dennison, G. J. Maddern. “Bioartificial liver support devices: historical perspectives”. ANZ Journal of Surgery. 73, 9, p.739-748 (2003).
[15] C. A. Vacanti. “The history of tissue engineering”. Journal of Cellular and Molecular Medicine. 10, 3, p. 569-576 (2006).
[16] G. K. Michalopoulos, M. C. DeFrances, Liver regeneration. Science 276, 60–66 (1997).
[17] "Workgroup on Expanded Criteria Organs for Liver Transplantation." Liver Transpl Liver Transplantation 11.10 (2005): 1184-192.
[18] J. P. Iredale, Models of liver fibrosis: Exploring the dynamic nature of inflammation and
repair in a solid organ. J. Clin. Invest. 117, 539–548 (2007).
[19] "Workgroup on Expanded Criteria Organs for Liver Transplantation." Liver Transpl Liver Transplantation 11.10 (2005): 1184-192.
[20] zamis, Maria-Louisa, Sinem Perk, Candice Calhoun, Korkut Uygun, Martin L. Yarmush, and François Berthiaume. "Machine Perfusion Enhances Hepatocyte Isolation Yields from Ischemic Livers." Cryobiology 71.2 (2015): 244-55.
[21] Bruinsma, Bote G., Tim A. Berendsen, Maria-Louisa Izamis, Heidi Yeh, Martin L. Yarmush, and Korkut Uygun. "Supercooling Preservation and Transplantation of the Rat Liver." Nat Protoc Nature Protocols 10.3 (2015): 484-94.
[22] Bruinsma, Bote G., James H. Avruch, Pepijn D. Weeder, Gautham V. Sridharan, Basak E. Uygun, Negin G. Karimian, Robert J. Porte, James F. Markmann, Heidi Yeh, and Korkut Uygun. "Functional Human Liver Preservation and Recovery by Means of Subnormothermic Machine Perfusion." Journal of Visualized Experiments JoVE 98 (2015): n. pag.
