Tissue Engineered Cartilage by Christine Davis and Sydney Phillipo
Articular Cartilage Damage
![Healthy cartilage vs osteoarthritic cartilage [13]](https://oww-files-public.sfo3.cdn.digitaloceanspaces.com/0/0d/Cartilage1.jpg)
Articular cartilage is the cartilage found between joints that reduces friction and acts as a shock absorber; it is flexible tissue composed of chondrocytes embedded in dense extracellular matrix (ECM)[1].Because this cartilage gets so much wear, some deterioration is inevitable. However, excessive deterioration caused by sports injuries, overuse, birth abnormalities, or osteoarthritis can be debilitating. Because cartilage is an avascular area, blood cannot bring nutrients, enzymes, or proteins to injured cartilage. Over 6 million people visit the hospital for cartilage damage every year, and osteoarthritis, or arthritis due to the degradation of articular cartilage, affects 33% of U.S. adults 65 years of age or older [2]. Osteoarthritis is a disease that breaks down articular cartilage and results in pain and loss of joint function. Osteoarthritis affects approximately 27 million Americans [3]. Health care costs related to cartilage damage are staggering. The total annual cost of living with osteoarthritis is $5,700/year per patient [4]. Job-related osteoarthritis costs are estimated at $3.4-$13.2 billion/year. Additionally, hospital related costs for total knee and hip replacements are estimated at $28.5 billion/year and $13.7 billion/year respectively. These statistics are merely for osteoarthritis and knee/hip replacements; they do not take into consideration various arthroscopic surgeries for cartilage debridement, tear suturing, etc., signifying that the potential market for an effective engineered cartilage is large and undertapped. The most limiting aspect of damaged articular cartilage is the absence of vascularization, which limits the blood and bone marrow to the cartilage tissue thus limiting repair and remodeling capabilities [1]. At the moment, we can repair cartilage but not fully restore it. The repairs have varying levels of efficiency and varying lifespans. Current engineered cartilage does not have the same properties of native cartilage and is insufficient. The goal is to one day be able to completely regrow new cartilage that perfectly matches the patient's needs, in size and immunocompatibility.
Cartilage Repair Issues
![Hyaline cartilage vs Fibrocartilage [14]](https://oww-files-public.sfo3.cdn.digitaloceanspaces.com/b/b9/Pic015.jpg)
Currently, scientists can easily grow chondrocytes, the cells native to cartilage, in a lab environment. The problem is with creating strong enough cartilage to withstand the immense pressures in vivo while making sure the chondrocytes stay in the areas they are needed. There is also a lack of research showing the effects of time on artificial cartilage function. The avascular nature of the cartilage means that any implanted cartilage must have all of the necessary factors to thrive, particularly in a low oxygen environment. Scientists cannot rely on blood vessels to bring oxygen, proteins, enzymes, growth factors, or any other building block to supply the engineered cartilage. Additionally, while many traditional treatments for cartilage damage do regenerate cartilage, the cartilage grown is not the ideal type. The type of cartilage needed for articular cartilage is called hyaline cartilage, and it is made of 5% chondrocytes and 95% extracellular matrix, mostly collagen and proteoglycans [2]. The type of cartilage formed by typical treatments is often a hybrid of hyaline cartilage and fibrocartilage, which has weaker mechanical properties. When cartilage is formed in vitro the cartilage is called neocartilage. There has not been any engineered cartilage made that contain the same mechanical properties as native cartilage, thus research is still be encouraged in this field.
History of Advances in Cartilage Repair
- 1743 : British anatomist, William Hunter, acknowledges the difficulties of cartilage repair leading the way for future research [6]
- 1965 : Chondrocytes first isolated and grown [7]
- 1970 : Willaim Green, MD performed experiments on potential of using autologous and homologous chondrocyte implantation for cartilage repair [6]
- 1984 : Lars Petersen, MD used treatments of patellar holes drilled in rabbit knees using autologous chondrocytes [7]
- 1985 : Peterson presented first report on optimizing cell delivery for cartilage repair to the Orthopedic Research Society [8]
- 1987 : 1st human trial using autologous chondrocyte implantation (ACI) was performed in Sweden [7]
- 1989 : Two famous articles published in the Journal of Orthopedic Research and Anatomical Record outlining the promising use of ACI [9]
- 1994 : Brittberg paper shows a majority of excellent or good ACI results of 23 patients where cartilage has hyaline-type appearance [10]
- 1995 : Autologous cartilage implantation begins to be widely used
- 1997 : Carticel® by Genzyme approved by the FDA for autologous chondrocyte implantation in knees as a second resort. This process has slight overgrowth problems, requires 2 surgeries (remove chondrocytes, send to Genzyme, re-implant with a periosteal patch from lower leg), not for patients with osteoarthritis [11]
- 2002 : Mitek Worldwide (Johnson & Johnson) and Verigen start MACI® clinical trials in the United States. MACI is similar to Carticel, but instead of a periosteal patch, a membrane of cow collagen is used. MACI allows for osteoarthritic patients and patients with large cartilage tears. [12]
- 2002-2010: Clinical trials begin for testing different hydrogels and matrices for ACI
- 2010: NeoCart starts clinical trials [13]
- 2012: MACI (Sanofi now), NeoCart proven to be superior to microfracture [13][14]
- 2014: Columbia University researchers grow human cartilage from mesenchymal stem cells [15]
- 2015: Columbia University researchers discover possible new stem cells that differentiate into chondrocytes [16]
- 2015: NeoCart reached phase III of clinical trials [17]
Cartilage Repair Techniques
Microfracture [2]
Microfracture is a common method for small repairs to articular cartilage. For this treatment small holes are created in the bone until it bleeds (cartilage is avascular, so this is the only way to introduce bleeding); this forms a matrix of blood clots that cartilage can begin to grow on . Most of the cartilage that forms is fibrocartilage and it is considered to be subpar. However, better cartilage repair is yielded in younger patients due to the abundance of mesenchymal stem cells that are responsible for repair. This approach is most appealing for its low morbidity rates, short recovery time, and minimal invasive procedure.
Osteochondral Autograft Transplantation (Mosaicplasty) [2]
Osteochoondral autografting is where healthy cartilage tissue are harvested from a healthy area on a patient and then implanted into the area of defect in order to restore cartilage function. Most often a “plug” of cartilage and bone is removed from one part of the knee and placed in the damaged part. Some of the problems encountered when using this technique include donor site morbidity, insufficient tissue, mismatching surface of the graft and the defect site, and the inability of the implanted tissue to withstand the weight of the new implanted site. The healthy tissue is generally taken from an low load-bearing area and required for a higher load-bearing area and cannot always handle the weight of that new region.
The autologous mosaicplasty technique is very similar, but in this case many small grafts are taken and implanted into the defect site, similar to a mosaic. This approach decreases donor site morbidity and can allow for a bettor contour of the implant site. Mosaicplasty has been shown to treat small cartilage defects, in the range of 1-4 square centimeters.
Osteochondral Allograft Transplantation [2]
Oseochondral allografting is similar to autografting. With this technique “plugs” are taken from a deceased donor (or tissue banks). This method eliminates donor site morbidity and lack of sufficient donor tissue. Since dead cells are being used, there are issues with maintaining the surface structure; this method also has limitations in contour matching and load-bearing capacity. The dead cells are also incapable of secreting proteins that natural cartilage secretes to lower friction between the bones thus there are issues with this treatment in some patients.
Autologous Chondrocyte Implantation (ACI) [2]
Autologous chondrocyte implantation procedure requires two surgeries. In this technique a small piece of healthy cartilage is removed from the patient's knee. Then, chondrocytes are taken from this tissue and grown in culture outside the body for 3-5 weeks. The second surgery involves the reimplantation of the cells into the patient underneath a periosteum flap (flap made of the patient's shin tissue to cover the chondrocytes over the defect site). This method has had satisfactory results for lesions of 1-12 square centimeters. The limitations of this technique include the long recovery time due to the nature of the surgeries, the time for cell culture, and the variation of chondrocyte cellularity in different patients. This method is approved for Genzyme and is referred to as Carticel. A similar procedure is available from Sanofi, called MACI, but instead of injecting the cells under a periosteum flap, it is injected into a membrane made of cow collagen.
Cell-Based Cartilage Resurfacing [19]
Cell-base cartilage resurfacing is similar to ACI in that it uses autologous cells to grow new cartilage. This treatments requires the extracting of chondrocytes from the patient's healthy cartilage tissue, which are then harvested (ex. NeoCart, though still phase 3). Once the cells are harvested they are embedded in a scaffold and reinserted into the defect area. The cells are implanted in a scaffold before reinsertion and incubated in a container that mimics cartilage formation conditions (low oxygen, varied mechanical forces). This technique can be used for fairly large cartilage defects. However, this method does take a long time due to the culturing of the cells. NeoCart is industry name for this method, created by Histogenics, and is currently in Phase III of clinical trials.
Cell Types Successfully Used
- Chondrocytes
Chondrocytes are the native cells of cartilage. They have been used in many methods of producing tissue engineering cartilage such as ACI, Carticel, NeoCart, and cell-based cartilage resurfacing. The main issue with using these cells is that they have limited repair capability, can be instable and it can be difficult to get donor tissue to retrieve these cells from[1]. They are most limited by their proliferation capacity.
- Mesenchymal Stem Cells [[1]]
Mesenchymal stem cells have recently been used due to their variability and in vitro capacity [19]. They have chondrogenic differentiation potential and are easily obtained making them ideal for cartilage repair.
- Periosteal cells
Periosteal cell come from the membrane covering the bone [19]. These cells can produce bioactive factors that are chondrogenic. Periosteum structures are used as part of Genzyme's Carticel method already, but the use of these cells could also be advantageous.
- Adipose derived stem cells (ADSCs)
ADSCs come from fatty tissue. In one study these cells were harvested from fat taken after a liposuctin surgery [20]. These cells are being considered due to their abundant proliferation in vitro, easy access, and their minimum immunological rejection.
Common Polymers Scaffolds Used [23]
- PLLA[[2]]
Polylactic acid is a biodegradable polymer that is commonly used in scaffolds. It was found, when porous, to be very effective in cartilage regeneration.
- PLGA
Poly (lactic-co-glycolic acid) is a biodegradable co-polymer. It is one of the most commonly used polymers in scaffolds because it is FDA approved.
- PDLA
Poly-D-lactic acid is another polymer used for it biodegradability. In studies for cartilage repair, it was one of the least effective at harvesting chondrocytes, but is still being researched for optimum use.
New or Specific Tissue Engineering Techniques
ACI-Derived Treatments
![NeoCart Process [15]](https://oww-files-public.sfo3.cdn.digitaloceanspaces.com/6/64/Cartilage2.jpg)
ACI-derived treatments center around injecting chondrocyte cells into patients. These cells have been previously harvested from the patients and grown in culture in a lab before being sent back to be reimplanted. The downside of these treatments is that they require two separate surgeries and require a significant amount of time [2].
- Carticel (by Genzyme in Cambridge, MA): cartilage cells are harvested from patient, sent to Genzyme, where they are grown and sent back. The cells are then injected into a fabricated pocket on the bone made of periosteal tissue from the lower limbs. This is a second option for patients who have tried more traditional methods, and it is not approved for use in osteoarthritic patients [11].
- MACI (by Sanofi in France): Similar to Carticel, cartilage cells are harvested from the patient, sent to Sanofi, where they are grown and sent back, but instead of a periosteal patch, a membrane of cow collagen is used. MACI is used in osteoarthritic patients and patients with large cartilage tears [12].
- NeoCart (by Histogenics in Waltham, MA): cartilage cells are harvested from patient, sent to Histogenics, where they are placed in a collagen scaffold and put in a proprietary incubator, which delivers various amounts of mechanical stress and oxygen levels [13]. This method is still in clinical trial and has not yet been approved.
Mesenchymal Stem Cell (MSC) Treatments
![MSC-promoted cartilage repair (Osiris)[12]](https://oww-files-public.sfo3.cdn.digitaloceanspaces.com/3/3e/Cartilage3.jpg)
MSC-derived treatments are important, because while autologous chondrocytes used via ACI are an effective treatment, articular cartilage repair requires many cells, sometimes too many to be harvested from other parts of the body. MSCs from bone marrow are the typical choice, as they are easily harvested, have good chondrogenic potential, and are most effective in vivo. To induce chondrogenesis, transforming growth factors, insulin-like growth factors, bone morphogenic proteins, fibroblast factors, varying hydrostatic pressure, varying cyclic compression, etc. can all be used in varying ways and amounts. Going forward, scientists seek to optimize culture conditions for MSC treatments, and to understand the differentiation mechanisms for MSCs. Most of the MSC-based treatments are still in clinical trials, but a few are in the final stages and show promise of being used in the near future [2].
- Chondrogen (Osiris): injections of chondrogen, which contains mesenchymal stem cells, are in the beginning phases of testing- they have been found to decrease the amount of osteoarthritis bone degeneration and pain [21].
- Cartiform (Osiris): cartilage mesh made of 3D hyaline cartilage with the necessary growth factors to stimulate MSC activity. MSC activity is passively stimulated (does not contain stem cells when implanted). Cartiform is currently in stage 3 clinical trials [22].
Embryonic Stem Cell(ESC) Treatments
ESC-derived treatments are nowhere near clinical trials, as ESC experimentation was tied up by political red tape for a long time. However, researchers have found that ESCs are able to form new cartilage with or without scaffolds. In the future, scientists will seek to improve differentiation efficiency and load-bearing aspects of the scaffolds [2].
Recent Experimental Studies
Comparison of Porous Scaffolds [23]
This experiment, performed in Japan, set out to determine what kind of scaffold would be most successful in harvesting chondrocytes to create engineered cartilage. By varying porosity and pore size they were able to determine how it effects the mechanical properties of the cartilage and an ideal porosity for future scaffolds. They used two different techniques to create the porous scaffolds; the first being fused deposition modeling (FDM) and the sugar leaching method (SLM). The scaffolds produced via SLM at varying pore sizes are shown in the image to the right. The compared scaffolds were made of PLLA, PDLA, PLA/CL, and PLGA and were infused with 10 million human articular chonrocytes per mL.
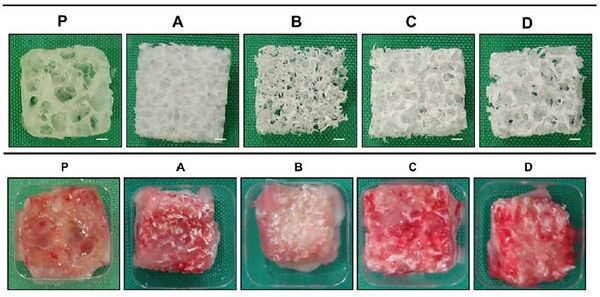
The results showed that PLGA produced the best cartilage of all scaffolds when at a porosity of 95% and a pore size of 0.3mm. However, PLGA did lose its shape after 2 months of being implanted, specifically on the edges. Ideally, the shape should not have changed because the rate of cartilage regeneration should be the same as the rate of biodegrading. The cartilage produced on the PLGA scaffold was similar to native cartilage in its white, smooth structure, but did lack in mechanical properties.
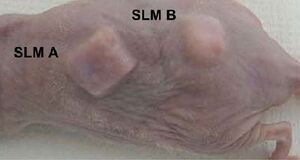
Engineered Cartilage for Ear Reconstruction [24]
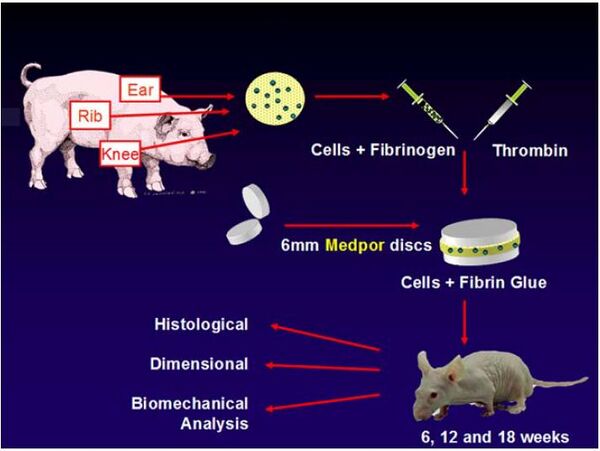
The main focus of this page has been the repair of articular cartilage, but this is not the only type of cartilage that has been researched. In 2014 research was conducted at Massachusetts General Hospital and Cornell University on the use of PPE for ear reconstruction. The chondrocytes used came from pigs knees, ribs, and ears. This was not the most ideal source for cells, but was enough for this preliminary experiment. These cells were then injected between two PPE discs to determine if the chondrocytes would be able to attached to the PPE and form cartilage around the 3D construct. The PPE used had a porosity of 50% and pore sizes between 100 and 400 micrometers. Typically, tissue engineered cartilage structures lose their 3D structure, but for the purpose of ear reconstruction it is important that it does not; this loss of structure was unsuccessfully analyzed in the experiment.
This experiment did show that cartilage would grow around the PPE, but since it was grown between two discs, mechanical properties could not be analyzed. There was a noticeable growth of cartilage after six weeks, with the entire trial running for a total of 18 weeks. Although this study showed how tissue engineered cartilage could be used for ear cartilage, it did not realistically show how it could be used. The idea is that the cartilage would not grow between to plates but would be able to form around a PPE scaffold and maintain its shape, thus allowing cartilage to grow in the shape of the ear.
Use of ADSCs on Hydrogel Scaffold [20]
This research took place in 2015 by researchers in China. For this experiment ADSCs were extracted from a middle-aged womans liposuction fat. These cells were then implemented into a chistosan/gelatin hydrogel scaffold and cultured in vitro for two weeks. The cell-embedded hydrogels were placed in a spinner flask bioreactor in order to culture the cells. Bioreactors are becoming an effective method of harvesting cells within the scaffold and was seen to be used in research in many fields. This bioreactor environment improved the cell growth and helped maintain the hydrogel's 3D structure. It was determined that the hydrogel would be a great prospect for future in vivo cartilage repair.
First Use of Stem Cells for Cartilage Repair
2014: Columbia University researchers published an article called "Large, Stratified, and Mechanically Functional Human Cartilage Grown In Vitro by Mesenchymal Condensation" in Proceedings of the National Academy of Sciences of the United States of America on April 28th, 2014 [15]. The article presented the labs groundbreaking procedure to induce adult MSCs to form human cartilage for the first time. So far, all treatments for cartilage damage using MSCs have merely stimulated, induced, or injected MSCs as treatment. This finding opens the door to potentially using MSCs to actually grow cartilage to implant in humans. Previous attempts to induce adult MSCs to form human cartilage added adult MSCs to hydrogels and cultured them with growth factors, nutrients, and mechanical loading procedures. However, this attempt nearly always produced mechanically weak cartilage. The researchers from Columbia decided to try to mimic the body's environment at the time this cartilage is biologically formed, so they first caused the MSCs to undergo condensation into cellular bodies. The findings from this procedure were that, for the first time, the strength and lubrication of the cartilage pieces was very close to that of native cartilage. This is an exciting discovery because it could lead to a future ability to be able to differentiate MSCs into cartilage.
Columbia University researchers form human cartilage from stem cells for the first time ever: <"https://www.youtube.com/embed/sJJ5920pqyQ">
2015: Columbia University researchers published an article called "Gremlin1 Identifies a Skeletal Stem Cell With Bone, Cartilage, and Reticular Stromal Potential" in Cell on January 15th, 2015 [16]. The article presented the prolific lab's findings of a new type of stem cell in mice that differentiates into bone and cartilage, called an osteochondroreticular stem cell, or OSC. It was previously believed that MSCs differentiated into bone and cartilage, but the mechanism was not known; MSC involvement could still be possible, or MSCs and OSCs could be different differentiations from the same line. Mice and humans have very similar bone-forming pathways, so these stem cells will likely be found in humans as well. After adulthood, OSCs only activate upon injury to support bone or cartilage; by learning to stimulate the differentiation of OSCs, scientists may be able to cause cartilage regeneration without any injections or in vitro culturing. This essentially complicates all previous research and methods, because if MSCs do not differentiate into cartilage, how have all of the other treatments been working? Much more research is needed to determine the implications of this.
References
- [1]Bhardwaj, N.; Devi, D.; Mandal, B. Tissue-Engineered Cartilage: The Crossroads Of Biomaterials, Cells And Stimulating Factors. Macromol. Biosci. 2014, 15, 153-182.
- [2] Zhang, Lijie, Jerry Hu, and Kyriacos A. Athanasiou. “The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration.” Critical reviews in biomedical engineering 37.1-2 (2009): 1–57. Print.
- [3]Brenner, J.; Kunz, M.; Tse, M.; Winterborn, A.; Bardana, D.; Pang, S.; Waldman, S. Development Of Large Engineered Cartilage Constructs From A Small Population Of Cells. Biotechnol Progress 2013, 29, 213-221.
- [4] "Osteoarthritis." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 16 May 2014. Web. 15 Feb. 2015.
- [5] Healthy Cartilage vs Arthritic Cartilage. Digital image. Osteoarthritis. Drugs.com, n.d. Web. 15 Feb. 2015.
- [6]Green WT Jr. Articular cartilage repair: behavior of rabbit chondrocytes during tissue culture and subsequent grafting. Clin Orthop Relat Res. 1977;(124):237-50.
- [7] Smith, Blair, Connie Li, Daniel Solomon, Matthew Whitson, Stephanie Chang. "Cartilage Repair History." Shoulder Repair for the Competitive Athlete. Brown University, 2 May 2004. Web. 15 Feb. 2015.
- [8]Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215-24.
- [9]Grande DA, Pitman MI, Peterson L, Menche D, Klein M. The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7(2):208-18.
- [10] Peterson, Lars, Olle Issakson, Claes Ohlsson, Anders Nilsson, Anders Lindahl, and Mats Brittberg. "Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation — NEJM." New England Journal of Medicine. New England Journal of Medicine, 6 Oct. 1994. Web. 15 Feb. 2015.
- [11] Genzyme Corporation. "Carticel (Autologous Cultured Chondrocytes)." (n.d.): n. pag. 2007. Web. 15 Feb. 2015.
- [12] Behrens, P., et al. "[New Therapy Procedure for Localized Cartilage Defects. Encouraging Results with Autologous Chondrocyte Implantation]." MMW, Fortschritte der Medizin 141.45 (1999): 49-51.
- [13] "NeoCart." Histogenics. Histogenics, n.d. Web. 15 Feb. 2015.
- [14] "Sanofi Biosurgery Product MACI Demonstrates Statistically Significant Clinical Outcomes Compared to Microfracture." Sanofi Press Releases. Sanofi-Aventis US, 12 July 2012. Web. 15 Feb. 2015.
- [15] Bhumiratana, Sarindr, et al. "Large, Stratified, and Mechanically Functional Human Cartilage Grown in Vitro by Mesenchymal Condensation." Proceedings of the National Academy of Sciences 111.19 (2014): 6940-5.
- [16] Worthley, D. L., M. Churchill, J. T. Compton, Y. Taylor, S. Mukherjee, and T. C. Wang. "Gremlin1 Identifies a Skeletal Stem Cell with Bone, Cartilage, and Reticular Stromal Potential." Cell 160.1-2 (2015): 269-84. Web. 15 Feb. 2015.
- [17]Noh, M. Orthopedic Cellular Therapy: An Overview With Focus On Clinical Trials. WJO 2015, 6, 754.
- [18] 3 Types of Cartilage. Digital image. Human Biological Science. Science1437- WestOne Services, n.d. Web. 15 Feb. 2015.
- [19]Gelse, K.; Mühle, C.; Franke, O.; Park, J.; Jehle, M.; Durst, K.; Göken, M.; Hennig, F.; Mark, K.; Schneider, H. Cell-Based Resurfacing Of Large Cartilage Defects: Long-Term Evaluation Of Grafts From Autologous Transgene-Activated Periosteal Cells In A Porcine Model Of Osteoarthritis. Arthritis Rheum 2008, 58, 475-488.
- [20]Song, K.; Li, L.; Li, W.; Zhu, Y.; Jiao, Z.; Lim, M.; Fang, M.; Shi, F.; Wang, L.; Liu, T. Three-Dimensional Dynamic Fabrication Of Engineered Cartilage Based On Chitosan/Gelatin Hybrid Hydrogel Scaffold In A Spinner Flask With A Special Designed Steel Frame. Materials Science and Engineering: C 2015, 55, 384-392.
- [21] Osiris. "Chondrogen." Therapeutics. Osiris Therapeutics, n.d. Web. 15 Feb. 2015.
- [22] Osiris. "Cartiform." Therapeutics. Osiris Therapeutics, n.d. Web. 15 Feb. 2015.
- [23]Tanaka, Y.; Yamaoka, H.; Nishizawa, S.; Nagata, S.; Ogasawara, T.; Asawa, Y.; Fujihara, Y.; Takato, T.; Hoshi, K. The Optimization Of Porous Polymeric Scaffolds For Chondrocyte/Atelocollagen Based Tissue-Engineered Cartilage. Biomaterials 2010, 31, 4506-4516.
- [24]O'Sullivan, N.; Kobayashi, S.; Ranka, M.; Zaleski, K.; Yaremchuk, M.; Bonassar, L.; Randolph, M. Adhesion And Integration Of Tissue Engineered Cartilage To Porous Polyethylene For Composite Ear Reconstruction. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2014, 103, 983-991.
- [25] NeoCart Proprietary Steps. Digital image. Histogenics. Histogenics, n.d. Web. 15 Feb. 2015.
