The BioBricks Foundation:BBFRFC16Draft
If BBFRFC16 is granted to me, then this is the draft for the standard.
Julie
The Protein Fusion Problem
BioBrick parts and standard assembly do not currently facilitate protein fusions. Since protein fusions are currently a small fraction of the parts synthetic biologists as a whole make, one approach is to simply decree that standard assembly does not support protein fusions. However, the community has developed new standards for making protein fusions, showing that they highly value the benefits of standard assembly. Now that these proposed standards have been created, a natural approach is to pick the best of these standards and convert every existing part over to it. However, protein fusions are unlikely to be the one construct that standard assembly and BioBrick parts do not support. Standard assembly needs to be made more flexible so that it can support a variety of improvements.
BioBricks and standard assembly do not allow the creation of protein fusions, as their composition prevents translation to continue directly from one protein part to another. The protein fusion standards proposed by the community allow one to compose two protein parts in a standardized fashion, but first require conversion or domestication of each part to the chosen standard (conversion to a standard is not always simple, even when converting a native part to a BioBrick part.) The family set of BioBrick parts introduced here, the BioScaffold parts, allow creation of protein fusions in many forms. They allow many protein parts in the registry to be directly translated into protein fusions in a customized fashion, with a standard methodology. At the same time, they incentivize standard forms for proteins that frequently fused with others such as protein tags. Thus, they satisfy the needs of both parts and device designers.
A Protein Fusion Strategy for Parts Designers: Using Off the Shelf Parts and Custom Linkers
Parts designers would like to take any two parts from the registry and connect them as they see fit. BioScaffold parts can enable this type of operation and are especially well suited to the construction of protein fusions by this method.
In order for protein parts to be compatible with this method, three requirements are placed on the parts. The protein parts should ideally begin with G (a BioBrick Prefix containing AT should precede them, completing the start codon) and end with TAA (instead of TAA TAA for instance.) See [ref] and appendix for a description of the BioBrick Prefix. Also the protein parts should not contain the PpiI restriction enzyme which is currently needed for removing protein tails. If the part contains a PpiI restriction enzyme site but not a PsrI restriction enzyme site, then the user should think about converting the part into a protein fusion part capable of automated assembly (see section below.) PpiI and PsrI have seven base pair recognition sites and thus are fairly rare. If the part has an unusual start (ATG) or end codon (TAA), then the user has three choices: see if can still work in the assembly, convert it to an appropriate protein fusion capable part (either for parts or device designers), or find a way to use it without modifying it using a combination of BioScaffold or BioMortar parts.
Example: Constructing a Protein Fusion Using BioBrick Parts and a Custom Linker
In order to place a custom linker between two BioBrick parts, first place a BioScaffold part between two BioBrick parts using standard assembly. (If you are using two protein parts and want to place a flexible linker between them you will need to use a BioScaffold part that removes protein tails, for instance BBa_J70030, or a part that removes both protein heads and protein tails, such as BBa_J70032. The vectors for the assembly process should be chosen to meet the requirements for 3A assembly.) See [ref] and appendix for DNA level descriptions of the BioBrick "Prefix," "Scar," and "Suffix" described below.
Table 2: Placing a BioScaffold part between two BioBrick parts First compose the prefix protein and BBa_J70030 using 3A assembly: <EcoRI cut Prefix w/AT>G protein TAA<SpeI cut Suffix> + <XbaI cut Prefix w/AG> BBa_J70030 <PstI cut Suffix>+<EcoRI and PstI cut Vector> Next compose the suffix protein with the composite prefix protein and BBa_J70030 part: <Prefix w/AT>G protein TAA<Scar w/AG> BBa_J70030 <SpeI cut Suffix>+ <XbaI cut Prefix w/AT>G protein TAA<SpeI cut Suffix>+<EcoRI and PstI cut Vector> Now you have a BioScaffold part composed between two BioBrick (protein) parts: <Prefix w/AT>G protein TAA <Scar w/AG> BBa_J70030 <Scar w/AT>G protein TAA<Suffix>
Now you will need to completely cut out and remove the BioScaffold part. Note that when the BioScaffold part is cut out, some adjoining sequence can be removed. (For example when using BBa_J70030, the protein tail and part of the protein head is removed.)
Table 3: Removing the BioScaffold Part
First you should cut with the BioScaffold part specific enzyme. (For BBa_J70030 it is PpiI.)
Location of PpiI cut sites in the assembly:
<Prefix w/AT>G . . . ppppp^TAA<Scar w/AG> BBa_J70030 <Scar w/AT> Gssss^. . . TAA<Suffix>
^ppppp ^Cssss^
This leaves BioScaffold part fragments that are too large to be removed with a Qiagen
PCR cleanup spin column. So first cleanup using the spin column and then cut with an
intermediate BioScaffold part enzyme. (For BBa_J70030 it is MabI or PacI.)
Then cleanup with a Qiagen PCR cleanup spin column to completely remove the BioScaffold part:
<Prefix w/AT>G . . . ppppp^ ^. . . TAA<Suffix>
^ ^Cssss
Now design a custom oligonucliotide part that will link together the two separate proteins. Usually the linker should code for a flexible linker (containing glycines and serines.) The main requirements here are to have an annealed oligonucleotide melting temperature greater than 30 degrees Celsius, so that the part can be ligated into the assembly, and a length than can be synthesized at reasonable cost (usually less than 60 base pairs.) It is also necessary to make sure that the protein translation will occur in frame after the linker.
Table 4: Creating a BioMortar flexible linker part to replace the BioScaffold part
Glycine-Serine-Glycine-Serine linker with a melting temperature of 38 degrees Celsius.
5'-GGT TCC GGG TC Gssss-3'
3'-ppppp CCA AGG CCC AG-5'
Gly-Ser-Gly-Ser
The oligo can now be annealed and ligated between the protein parts (restoring them to their orginal form with a flexible linker joining them.)
Table 5: The final construct
<Prefix w/AT>G protein GGT TCC GGG TCG protein TAA<Suffix>
CCA AGG CCC AGC
Gly-Ser-Gly-Ser
A Protein Fusion Strategy for Device Designers: Automated Assembly and Defined BioBrickPF Parts
Protein designers do not want to have to design a custom part every time they wish to make a protein fusion. They want to be able to take protein parts off the shelf and be able to immediately put them together into protein fusions. This may mean that they will work with different types of parts than protein designers. For instance they may place a premium on well tested parts that have been used in creating protein fusions before when designing their system. Ideally, creating a part that can be used by device designers should be the last step in the design process by a part designer. When a part is handed off in this form, the device designer will gain an additional measure of confidence in using the part in the future.
Format of BioBrickPF parts
Prefix Protein Fusion Preparation Part (BioBrickPF-P): Composition of a BioBrick protein part and a BioScaffold part that can be cut with the BioScaffold part specific enzyme, leaving characteristic ends.
Table 6: Format of the BioBrickPF-P (protein and BioScaffold) composite parts BioBrickPF-P(Type 1) Part:(Prefix Protein has a tail, BioScaffold specific enzyme is PpiI) <Prefix w/AT>G protein GGATCC TAA <Scar w/AG> BBa_J70030 (PpiI) <Suffix> BioBrickPF-P(Type 2) Part:(Prefix Protein lacks a tail, BioScaffold specific enzyme is PpiI) <Prefix w/AT>G protein GGATCC <Scar w/AG> BBa_J70010 (PpiI) <Suffix> BioBrickPF-P(Type 3) Part:(Prefix Protein lacks a tail, BioScaffold specific enzyme is PsrI) <Prefix w/AT>G protein GGATCC <Scar w/AG> BBa_J70012 (PsrI) <Suffix>
Table 7: Format of the BioBrickPF-P parts after excision with the BioScaffold specific enzyme:
<Prefix w/AT>G protein G GATCC^
C^
Suffix Protein Fusion Preparation Part (BioBrickPF-S): Composition of a BioBrick protein part and a BioScaffold part that can be cut with the BioScaffold part specific enzyme, leaving characteristic ends.
Table 8: Format of the BioBricPF-S (BioScaffold and protein composite) parts BioBrickPF-S(Type1) Part:(Suffix Protein has a head, BioScaffold specific enzyme is PsrI) <Prefix w/AG> BBa_J70012 <Scar w/AT>G GGATCC protein TAA<Suffix> BioBrickPF-S(Type1) Part:(Suffix Protein lacks a head, BioScaffold specific enzyme is PpiI) <Prefix w/AG> BBa_J70010 or BBa_J70030 <Scar w/AG> GATCC protein TAA<Suffix>
Table 9: Format of the BioBrickPF-S parts after excision with the BioScaffold specific enzyme:
^ protein TAA<Suffix>
^CTAGG
The current BioScaffold parts enable automated 3A assembly using BioBrickPF-P and BioBrickPF-S parts since they can be heat killed and removed by Qiagen PCR spin columns or other purification techniques depending on the size of the proteins []. This allows both the BioScaffold parts and appropriate BioBrick ends on the prefix and suffix proteins to removed so that the the protein fusion parts can be ligated into a vector containing a different antibiotic resistance than the original BioBrickPF-P and BioBrickPF-S part containing vectors.
Table 10: Composing BioBrickPF-P and BioBrickPF-S parts (using 3A assembly and after excision of the
BioScaffold parts)
Assembly:
<EcoRI cut Prefix> protein G GATCC^ + ^ protein TAA <PstI cut Suffix>+<EcoRI and PstI cut Vector>
C^ ^CTAGG
Final construct:
<Prefix> protein GGA TCC protein TAA <Suffix> <Vector>
CCT AGG
Gly-Ser
The Library Construction Problem
The goal is to be able to introduce new parts (often with a variable region) into an assembly as the last step in the construction process.
Library Construction Using BioScaffold β parts
Cut within the scars
BioScaffold Interior Parts
^pppp pppp GCAGGTG GACAAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC ssss^ssss
pppp^pppp CGTCCAC CTGTTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG ssss ssss^
AarI site XmaI site AarI site
Vector Preparation
<Prefix> part1 <Scar> BBa_JX <Scar> part2 <Suffix>
<Prefix> part1 TACTAGAT BBa_JX TACTAGA(T or G) part2 <Suffix>
ATGATCTA ATGATCT(A or C)
<Prefix> part1 ^TACT AGAT BBa_JX TAC^TAGA (T or G) part2 <Suffix>
ATCA^TCTA ATG ATCT^(A or C)
<Prefix> part1 ^ ^TAGA (T or G) part2 <Suffix>
ATCA^ ^(A or C)
BBa_JX= BBa_J70034
^TACT AGAT GCAGGTG GACAAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC A TAC^TAGA
ATGA^TCTA CGTCCAC CTGTTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG T ATG ATCT^
AarI site XmaI site AarI site
<Prefix w/AT or AG> GCAGGTG GACAAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC A <Suffix>
CGTCCAC CTGTTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG T
Insert Preparation <Prefix> BBa_JY <Scar> insertpart <Scar> BBa_JY <Suffix>
<Prefix> BBa_JY TACTAGA(T or G) insertpart TACTAGAG BBa_JY <Suffix>
ATGATCA(A or C) ATGATCTC
<Prefix> BBa_JY ^TACT AGA(T or G) insertpart TAC^TAGA G BBa_JY <Suffix>
ATGA^TCA(A or C) ATG ATCT^C
^TACT AGA(T or G) insertpart TAC^
^TCA(A or C) ATG ATCT^
The vector and the insert or inserts can now be ligated together to create the final part.
<Prefix> part1 ^TACT AGA(T or G) insertpart TAC^TAGA (T or G) part2 <Suffix>
ATCA^TCA(A or C) ATG ATCT^(A or C)
BBa_JY
TAC^TAGA (G or T) ATA GCAGGTG GACAAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC ATAT ^TACT AGA(G or T)
ATG ATCT^(C or A) TAT CGTCCAC CTGTTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG TATA ATGA^TCT(C or A)
<Prefix> ATA GCAGGTG GACAAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC ATAT <Suffix>
TAT CGTCCAC CTGTTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG TATA
tactagat ^TACT AGAT PI1 TAC^TAGA (T or G)
ATGA^TCTA ATG ATCT^
tactagag ^TACT AGAG PI1 TAC^TAGA (T or G)
ATGA^TCTC ATG ATCT^
will these anneal? no
^ ^TAGA
ATGA^ ^
Preparation parts
(PI2 Left) ^TACT AGAT library insert TAC^TAGA (G) (PI3 Right)
ATGA^TCTA ATG ATCT^(C)
Another way but may anneal
TA^CTAG AT library insert ^TACT AGA(G)
AT GATC^TA ATGA^TCT(C)
For parts preparation
TACTA^GAG B1 TA CTAGA^G ^ATGAT CTC part to remove AT^GATCA C
TACTA^ ^ ^ ^GATCA
For final introduction
TA CTAGA^G Part ^TACTA GAG AT^GATCA C ATGAT^CTC
<Prefix><Psr I><Construct><Scar w/AG><PpiI><Scar w/AG><PsrI><Suffix> <Prefix><Psr I><
B2left TACTA^GAG part to insert TA CTAGA^G
^ATGAT CTC AT^GATCT C
Library Construction Using BioScaffold α parts
First place a BioScaffold part between two BioBrick parts as shown in Table 2.
You do not continue directly along the lines of the protein fusion protocol for parts designers, because the digestion efficiency of the BioScaffold specific enzymes PpiI and PsrI are below 95 percent which is not ideal for library construction, where you wish to retain as much diversity (or complexity in the library as possible) and this inefficiency would reduce the number of clones produced.
Remove the BioScaffold part as appropriate for that part.
<Prefix> . . .ppp ppppp^ ^ssss. . . <Suffix>
ppp^ ^Cssss ssss
Replace it with a Peisajovich inspired BioMortar part (this type of part is inspired by Sergio Peisajovich's AarI assembly standard developed with the UCSF 2007 iGEM team and Wendell Lim.) Then sequence to select appropriate clones.
Peisajovich inspired BioMortar part (Melting Temperature greater than 76 degrees Celsius)
5'- GCAGGTC GACAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC Gssss-3'
3'-ppppp CGTCCAC CTGTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG -5'
AarI site XmaI site AarI site
<Prefix w/AT>G . . . ppppp GCAGGTC GACAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC Gssss . . . TAA<Suffix>
ppppp CGTCCAC CTGTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG Cssss
Digest with AarI and XmaI and purify according to the Peisajovich AarI digestion protocol [], yielding the following product.
<Prefix w/AT>G . . .^pppp pppp GCAGGTC GACAGAGGAGT CCCGGG AGCTGGAACTCC CACCTGC Gsss^ssss s . . . TAA<Suffix>
pppp^pppp CGTCCAC CTGTCTCCTCA GGGCCC TCGACCTTGAGG GTGGACG Csss ssss^s
The Parts Domestication Problem
Currently, it is not yet clear whether nonstandard BioBrick parts will be sequenced, maintained, or distributed by the Registry of Standard Biological parts in the long term. Nonstandard parts are problematic for the registry because they impose additional costs on the user. However, in cases when the BioBrick standard could not meet all the needs of the user, nonstandard parts have sometimes been allowed. BioScaffold parts, by building upon the work of the community, suggest a way in which the need for these nonstandard parts can be minimized.
nonstandard parts are not allowed in the registry. This is good because it means that parts can be used interchangeably and that the parts included in the Registry distribution are high quality parts.
However, sometimes there are reasons why a BioBrick parts user, especially a parts designer or someone from outside the synthetic biology community, may not want to immediately convert a part to the BioBricks standard.
-There is not yet a one step protocol for converting more than 5 restriction in a part over to the BioBrick standard though (http://openwetware.org/wiki/Knight:Site-directed_mutagenesis/Multi_site provides a protocol for up to 5 sites at a time.) Creating a process that routinely achieves conversion in one step for up to 10 sites would be an excellent BBF RFC standard.
-They may be publishing a paper and want to direct people who want the part to the Registry both to share their part and in order to attract more citations for their paper, however, they do not want to delay publication of their paper.
-For some technical/functional reason converting their part to the BioBrick standard does not make sense. In this case the reason should be reported to the community in a BBF RFC so that the community can try to find a way to fix the problem.
-They wish to donate a large collection of parts to the Registry.
-They would like to try out a new standard for a certain part of the assembly process and have not yet found a way to integrate their new standard with the BioBricks standard (we hope that the new BioScaffold parts will help facilitate integration.)
Philosophy
Reasons to allow non-BioBrick parts into the registry
-Registry and collection of parts will grow more quickly
-With BioScaffold parts, non-BioBricked parts can be placed in BioBricked vectors with minimal effort such that they can be sequenced, maintained, and distributed by the Registry
-BioScaffold parts now make it possible to integrate non-BioBricked parts into BioBricked constructs (as described below) -Allows parts designers greater freedom in manipulating parts and testing options before selecting the optimal part
-Divergent standards will not cause fragmentation of the community due to different parts only being held in multiple divergent registries
-Paper authors can submit their non-BioBricked parts to the registry before publication to satisfy materials distribution requirements and attract additional citations to their papers, however at the same time their path to publication will not be slowed
However non-BioBrick parts impose unnecessary costs on the user, and across the whole community the costs become significant
-incompatible with existing parts since do not allow standard assembly
-require additional sequencing (each part must be sequenced fully when prepared by pcr, rather than just be verified)
-lead to storage of more instances of the part in the registry
-the BioScaffold enzymes are not as efficient as the BioBrick enzymes, and manipulations with them are more time consuming
Thus, the community should incentivize conversion from BioBrick parts to non-BioBrick parts as soon as possible A few possibilities -Create a non-standard BioBrick part sandbox, maintained by the Registry-- parts in this sandbox will not be distributed until someone claims the conversion rights for the part-- they then have 6 months to convert the part and return it to the registry
-Create a BioBrick part journal as part of the parts promotion process, and not allow publication until conversion -After conversion, credit both the part creator and part converter as authors in the BioBrick parts journal -Allow people to claim conversion rights on the registry's
B-S enzymes available
PsrI, PpiI, AarI
<BS
Always want to add your undomesticated parts last to the assembly?
<BS-Non-Cutter> <Part> <Part> <Part> <Part><BS Cutter><BS-Non-Cutter>
<BS-Non-Cutter> <Part> <Part><BS-Cutter><Part> <Part><BS-Non-Cutter>
<BS-Non-Cutter><BS-Cutter> <Part> <Part><Part> <Part><BS-Non-Cutter>
<Prefix Scar>
<BS-Non-Cutter><Scar><UD Part><Scar><BS Non-Cutter>
<Scar Suffix>
<Scar Scar>
<Scar> tactagag or tactagat(g)
<Prefix> gaattcgcggccgcttctagag or gaattcgcggccgcttctagat(g)
<Suffix> tactagtagcggccgctgcag
Allowing a construct to be transferred to a new vector, while removing the BioScaffold parts (<B-P> parts)
Old vector
<Prefix> <B-P><Scar> construct<Scar> <B-P> <Suffix>
gaattcgcggccgctt ctaga g ta ctaga^(t or g) tacta^gag tacta gtagcggccgctgcag
^ ^
New vector
<Prefix> <B-P part sealer>
gaattcgcggccgctt ctaga^g tacta^gtagcggccgctgcag
^ ^
<Prefix> <B-P1 A> <Scar> construct <Scar> <B-P2 A> <Suffix>
gaattcgcggccgc ttcta^gag ta ctaga^(t or g) tacta^gag ta ctagt^agcggccgctgcag
^ ^ ^ ^
Left part vector insertion vector <Prefix><B-P3 A><Scar>xx ttcta^gag<B-P1 A><Scar> <B-P beta> <Scar><B-P3><Suffix>
Right part vector insertion vector <Prefix><B-P4><Scar><B-P beta><Scar><BP-2>ta ctagt xxx <Scar><BP-4><Suffix>
Part insertion pcr format <B-P beta><Scar>part<Scar><B-P beta> Part insertion oligo format XXXXXXXXX
<BioScaffold PsrI> <Scar> construct <Scar> <BioScaffold PpiI> <Scar> <BioScaffold PsrI>
<BioScaffold PsrI> <Scar> Part <Scar> <BioScaffold PsrI>
<BioScaffold PsrI> <Prefix> construct <Scar> <BioScaffold PpiI> <Suffix> <BioScaffold PsrI>
<BioScaffold PsrI> <Scar> Part <Suffix> <BioScaffold PsrI>
Current Suite of BioScaffold Parts and Functional Examples of their Use
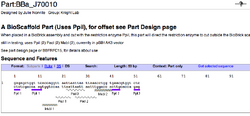
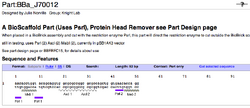
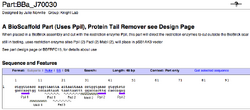
To demonstrate the utility of BioScaffold parts the current designed BioScaffold parts in the Registry (Figure 7) and examples of their potential use are included (Figure 8).
| Figure 7: Examples of BioScaffold Parts | ||
|---|---|---|
 |
 |
 |
| Figure 8: How They Work | ||
 |
 |
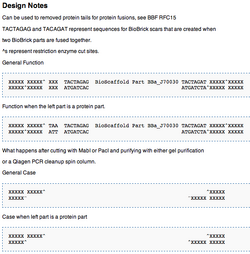 |
Specific Examples of BioScaffold Parts and Their Compatibility With the Registry
The specific examples of BioScaffold parts (Figure 7, 8) and their applications are intended to demonstrate how BioScaffold parts can increase the capabilities of standard assembly in a manner that can sustainably foster the evolution of standard assembly to acquire new capabilities. The community is also encouraged to create new BioScaffold parts, applications for BioScaffold parts, optimized protocols for the parts, and to help enact the minimal infrastructural changes that allow nearly universal use of the parts.
The primary way in which the BioScaffold parts are incompatible with the registry is that some plasmids contain one to two recognition sites of the enzyme used to cut the BioScaffold part out of the assembly. The PpiI enzyme which is used to cut out two types of BioScaffold parts is the primary culprit. The most pressing issue to facilitate the adoption of BioScaffold parts is to remove PpiI sites from commonly used BioBrick plasmids pSB1AC, pSB1AT, and pSB1AK. The restriction sites PsrI, MabI, PacI, and AscI were rarely present.
| Plasmid | Enzyme | Number of Recognition Sites | Commonly Used Plasmid |
|---|---|---|---|
| pSB1A3, discontinued 18 | PpiI | 2 | No |
| pSB1AC3, high copy 19 | PpiI | 2 | Yes |
| pSB1AK3, high copy 19 | PpiI | 2 | Yes |
| pSB1AT3, high copy 19 | PpiI | 2 | Yes |
| pSB1A7, transcriptionally insulated high copy 19 | PsrI and PpiI | 2 each | Sometimes |
| pSB2K3, use pSB2K4 instead | AscI | 1 | No |
| pSB2K4, inducible copy 19 | None | None | Yes |
| pSB3C5, low to medium copy 19 | PpiI | 1 | Yes |
| pSB3K3, probably discontinued | PpiI | 1 | No |
| pSB3T5, low to medium copy 19 | PpiI and PsrI | 1 each | Yes |
| pSB4A3, discontinued 18 | PpiI | 1 | No |
| pSB3K5, low to medium copy standard vector 19 | PpiI | 1 | Yes |
| pSB4C5, low copy standard vector 19 | None | None | Yes |
| pSB4K5, low copy standard vector 19 | None | None | Yes |
| pSB4A5, low copy standard vector 19 | PpiI | 1 | Yes |
| pSB4T5, low copy standard vector 19 | PsrI | 1 | Yes |
To use BioScaffold parts to create a protein fusion, to develop a library of parts, or to domesticate a non-BioBricked part one must first compose them with other parts of interest using standard assembly. As shown (see Figure 7), the BioScaffold parts are no different from any other BioBrick parts in the Registry either in how they are represented or how they can be inserted into an assembly. Two existing BioScaffold parts demonstrate a number of principles of BioScaffold design. One part, BBa_J70012 in the Registry, is based upon the restriction enzyme PsrI and the other BBa_J70010 is based upon the restriction enzyme PpiI. (Notice: BBa_J70012 and BBa_J70010 have been constructed, sequenced, and are expected to be distributed with the next version of the Registry to iGEM teams.) These BioScaffold parts gain their utility from the Type IIB restriction enzymes, which allow them to cut around BioBrick scars (see Figure 1 and Figure 5) in a positionally sensitive matter. This means that the PsrI BioScaffold part BBa_J70010 can cut around the protein head or ATG + 1 sequence commonly found at the beginning of a protein, regardless of the identity of the protein start (see cut sites in Figure 8.) But how can one cut around the TAA tail of a protein, since the way to do this is not clear from the cut sites of either part in Figure 8? A new BioScaffold part with this property can easily be created as the cut site on the left side of BBa_J70012 can easily be shifted 3 base pairs to the left, creating a new BioScaffold part, since the right most 3 base parts on the scar sequence are the same as the left 3 base pairs of the recognition site of PpiI (see Figures 7a, 8a, and 9.) Thus, BioScaffold parts can be useful in creating protein fusions. The restriction enzymes PsrI and PpiI were selected for the rarity of their cut sites and their ability to create cuts offset from a reconition site. Though some high copy and low copy plasmids in the Registry of Standard Biological Parts contain the cut sites, most do not require more than two base pair changes per vector to remove the sites (note for every two cuts, only one modification is required since the Type IIB restriction enzymes are bipartite.) The greatest flaw of these versions of the BioScaffold parts is that the restriction enzymes used are less efficient in cutting and religation than the traditional BioBrick enzymes. The BioScaffold parts described here contain a number of internal restriction enzyme cut sites (for example for MabI, AscI, and PacI.) The current BioScaffold parts are rather long and the additional restriction enzyme cut sites provide a choice about how to cut the part so that it can be removed by Qiagen spin column purification in some applications (note: double stranded oligonucleotides less than 40 base pairs can be removed with the Qiagen kit.) The cut sites also help prevent religation of the parts in the case where only one TypeIIB restriction enzyme is active The internal cut sites also allow the BioScaffold to can be cut during ligation as a form of error correction. When using the polymerase chain reaction to prepare a BioMortar part, only one recognition site for either PsrI or PpiI (rather than the whole BioScaffold part) can be added to each amplification oligonucleotide at the correct position. This allows one to still use a part that contains one of these restriction enzymes but not the other. Each of the enzymes used in the design of these parts (PpiI, PsrI, MabI, AscI, and PacI) can be heat killed, meaning that the use of BioScaffold parts is compatible with automated assembly.
References:
[1] Tom Knight, Randall Rettberg, Leon Chan, Drew Endy, Reshma Shetty, and Austin Che "Idempotent vector design for standard assembly of BioBricks," BBF RFC 9, 2003, <http://bbf.openwetware.org/RFC.html.>
[2] Tom Knight, "Draft standard for BioBrick biological parts," BBF RFC 10, May 3, 2007, <http://bbf.openwetware.org/RFC.html.>
[3] Austin Che, "BioBricks++: Simplifying Assembly of Standard DNA Components," June 9, 2004, <http://austinche.name/docs/bbpp.pdf>
[4] Ira Phillips and Pamela Silver, "A New Biobrick Assembly Strategy Designed for Facile Protein Engineering," MIT SBWG Technical Reports, April 20, 2006, <http://dspace.mit.edu/handle/1721.1/32535>
[5] Tom Knight, "Biobrick assembly standard modification," BBF RFC 11, July 8, 2008, <http://bbf.openwetware.org/RFC.html.>
[6] Tom Knight, "Draft BB-2 standard for biological parts," BBF RFC 12, November 19, 2008, <http://bbf.openwetware.org/RFC.html.>
[7] Tom Knight, "Rethinking the boundaries and composition of coding regions," BBF RFC 13, November 19, 2008, <http://bbf.openwetware.org/RFC.html.>
[8] Tom Knight, "Protein domain fusions in BB-2 assembly," BBF RFC 13, November 23, 2008, <http://bbf.openwetware.org/RFC.html.>
[9] BioBricks Foundation: Standards/Technical/Formats <http://openwetware.org/wiki/The_BioBricks_Foundation:Standards/Technical/Formats#The_Berkeley_.28BBb.29_Format>
[10] Sergio Peisajovich, Wendell Lim, and 2007 UCSF iGEM team, October 30, 2008, <http://2008.igem.org/Team:UCSF/Materials_and_Methods.>
[11] Chris Anderson and Zhan Jian, Plasmids/Library Screening, <http://partsregistry.org/Plasmids/Library_screening.>
[12] Reshma Shetty and Randall Rettburg, "3A Assembly," December 13, 2007, <http://openwetware.org/wiki/Synthetic_Biology:BioBricks/3A_assembly.>
[13] Kristian Müller and Katja Arndt, "Restriction sites for the construction of fusion proteins," BBF RFC 3, <http://bbf.openwetware.org/RFC.html.> (Note: this RFC is not yet posted, but is expected to describe the standard in [9].)
[14] Julie Norville, "Part: BBa_J70010, A BioScaffold Part," Registry of Standard Biological Parts, May 5, 2008 <http://partsregistry.org/Part:BBa_J70010.>
[15] Julie Norville, "Part: BBa_J70012, A BioScaffold Part," Registry of Standard Biological Parts, May 5, 2008 <http://partsregistry.org/Part:BBa_J70012.>
[16] Julie Norville, "Part: BBa_J70030, A BioScaffold Part," Registry of Standard Biological Parts, December 12, 2008 <http://partsregistry.org/Part:BBa_J70030.>
[17] Randall Rettberg and others, "Help: BioBrick Prefix and Suffix," Registry of Standard Biological Parts, July 23, 2006 <http://partsregistry.org/Help:BioBrick_Prefix_and_Suffix.>
[18] Reshma Shetty, "Plasmid Backbones/Archive," Registry of Standard Biological Parts, December 11, 2008 <http://partsregistry.org/Plasmid_backbones/Archive.>
[19] Reshma Shetty, "Plamid Backbones/Assembly," Registry of Standard Biological Parts, December 11, 2008, <http://partsregistry.org/Plasmid_backbones/Assembly.>