Stents by Shayna Nolan, Abigail Sossen and Ryan Colombo
Introduction
Coronary Heart Disease (CHD), also referred to as Coronary Artery Disease (CAD), is the leading cause of death
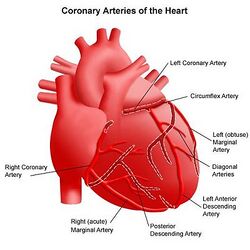
for both men and women in the US [1]. CHD is caused by the accumulation of plaque on the arterial walls, and may also be caused by the hardening of the arteries. Plaque is composed of lipids/triglycerides, calcium deposits, white blood cells, and cellular debris [2].Over time, the buildup of this fatty plaque causes the arteries to narrow, which can slow or even inhibit blood flow to the heart [1]. The occlusion of the coronary arteries can lead to angina (chest pain) and heart attack (myocardial infarction). Risk factors for the disease include: high LDL or “bad cholesterol” (triglycerides in the blood), low HDL or “good” cholesterol, high blood pressure (hypertension), smoking, obesity, lack of exercise, stress, diabetes, poor diet, depression and family history [3]. Medical treatments for heart disease, high blood pressure, or high cholesterol in combination with lifestyle changes can treat mild CHD. For more advanced cases, however, surgical procedures such as percutaneous coronary intervention or coronary artery bypass may be required [1].
The progression of coronary artery disease is about 40 to 60% based on genetic variables. 33 genetic variants have been linked to the development and progression of coronary artery disease most of which are SNPs (single nucleotide polymorphisms). Of these 33, 8 are related to lipid production, 2 are related to hypertension and 23 are still unknown. Most of these are non-protein coding sequences (introns) thought to be regulatory regions known as long noncoding RNAs (lncRNA). More genetic risk factors correlate to more aggressive and earlier onset coronary artery disease. A current research goal is to better understand the mechanisms by which these genetic variants lead to coronary artery disease development and progression [4][5][6].
Angioplasty and Stent Implantation
An angioplasty is a procedure that restores blood flow to narrow or blocked arteries [7]. In an angioplasty, imaging techniques are used to direct a balloon-tipped catheter into an artery or vein [7]. Once the site of blockage is reached, the balloon is inflated to open the vessel, deflated, and removed. A stent may be placed around this balloon, which is deployed upon balloon inflation and will maintain the opened artery [7]. This balloon can be inflated to different sizes depending on the size of the vessel.
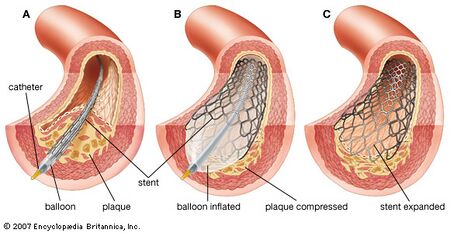
Angioplasty with stenting is initiated when a nick is made at the site of insertion. A sheath is inserted into the artery, into which the catheter is inserted and guided using fluoroscopy to the blocked site. Fluroscopy converts x-rays into video images that the doctors can see [7]. A thin guide wire is used to place the balloon-tipped catheter at the blocked site. Next, the balloon is inflated, which pushes the stent against the arterial wall. The stent remains in place once the balloon is deflated [7]. Following successful deployment, the catheter is removed and bleeding is stopped using pressure. No sutures are needed, although a closure device may be required to seal the small hole in the artery [7].
Angioplasties were an advantageous alternative to bypass surgery and medical therapies for treatment of mild coronary artery disease. However, post angioplasty, 1/3 of patients developed progressive or refractory angina, which required invasive treatment [8]. In addition, 5% of patients developed acute vessel closure or flow-limiting dissection, and 1/5 of patients required additional procedures over the next 3 years [8]. Therefore, a need for a device that could maintain lumen integrity and prevent reclosure of the vessel led to the development of the stent.
Uses
Stents are used to treat stable coronary artery disease, in which a drug-eluting stent is used in conjunction with an antiplatelett therapy [9]. Atherosclerosis, or the hardening of arteries, occurs when fat or cholesterol accumulates on the arterial walls, forming plaques. This is commonly associated with diabetes. Multivessel disease and Left Main Coronary Artery disease are also treated using stents [9].
Bare Metal Stents
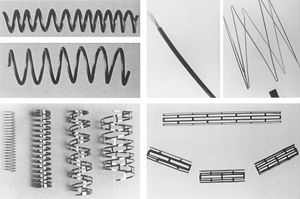
Bare metal stents have been developed in three main designs: coil, tubular mesh, and slotted tubes. The coil stents have been phased out but once consisted of a piece of stainless steel formed into a coil. The tubular mesh was successful consisting of wires forming a meshwork to form a tube. Most modern stents now follow the slotted tube design in which the strut design is cut using lasers Bare metal stents were once made out of only stainless steel, now cobalt chromium, titanium chromium, titanium nickel, nickel chromium and platinum chromium alloys have all been used successfully [10].
Although they are still used today, bare metal stents are associated with several severe complications that has prompted the development of drug eluting stents. One of the major problems with bare metal stents is the occurrence of neointimal hyperplasia [9,13]. Neointimal hyperplasia is the growth of scar tissue within the stent due to a proliferation and migration of smooth muscle cells in response to strut-related inflammation [13]. This leads to restentosis, or a re-narrowing of the blood veseel, and requires revascularization. Restenosis has occurred in 20-30% of patients treated with bare metal stents, usually within 5-6 months after treatment [8,9,13]. Endothelial injury caused by bare metal stents can also render vessels thromogenic [11]. Thrombosis occurs when a blood clot forms within a blood vessel, and is lethal in 45% of events[12]. In addition, the fibrinoogen layer covering the metal stent surface can induce platelet activation and thrombosis, which must be countered using anti-platelet medicines. Dual antiplatelet therapies consist of aspirin and clopidogrel or plavix, and are administered for 6 - 12 months [11].
Drug Eluting Stents
Drug eluting stents (DES) are characterized by the controlled release of immunosupressive and antiproliferative agents, which act to inhibit the accumulation of smooth muscle cells [9,15]. These stents were developed to counter restenosis, a complication of their bare metal predecessors. DES's consist of three components: the metal stent, polymer coating, and eluting drug [16]. The polymer coating on the stent enables controlled drug release, and must be biocompatible in order to decrease local inflammatory reactions and thrombosis [9]. In 2012, drug eluting stents were implanted in more than 500,000 patients in the US [9]. Drug eluting stents have been deemed more cost effective than bare metal stents, as the higher cost of these stents is offset by the reduced need for revascularization procedures [16].
First Generation Drug Eluting Stents
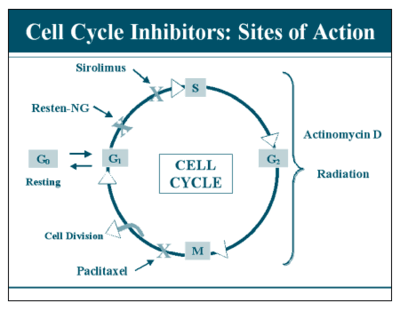
First Generation Drug eluting stents elute one of two major drugs (paclitaxel or sirolimus) which both act as cell cycle inhibitors to prevent proliferation and induce apoptosis. The stents are usually coated with a polymer that acts as a drug delivery agent, the drugs are slowly released for up to one year after the stent has been implanted. It has yet to be determined whether or not a bioresorbable polymer is good for drug delivery, because eventual contact between the metal and the blood will occur which may increase the risk for restenosis [17]. These antiproliferative drugs reduce revascularization as a result of neointimal hyperplasia. Similar risks of death and myocardial infraction exist as compared to BMS's [1]. With regard to structure, first generation DES's are identical in structure to their stainless steel BMS predecessors, with strut thicknesses of 130-140 μm [18].
Paclitaxel
Paclitaxel, a compound isolated from the bark of the Pacific Yew tree, inhibits microtubule disassembly during cell replication [19]. Like Sirolimus, it is used in cancer treatments and can inhibit smooth muscle cell proliferation. Boston Scientific manufactures the Paclitaxel-eluting Taxus stent, a stent constructed from 316L stainless steel with 132 μm struts and a Transulate® polymer coating [poly(styrene-b-isobutylene-b-styrene)] [19]. Significant reductions in restenosis and revascularization have been obtained with the Taxus stent.
Sirolimus
Sirolimus, or Rapamycin, was discovered on Easter Island as an antifungal agent [20]. Its antiproliferative properties have enabled its use in cancer therapy and transplant rejection therapy. When eluted from a stent, Sirolimus inhibits the proliferation of smooth muscle cells and leukocytes. The Cypher stent, a Sirolimus-eluting stent, is manufactured by Cordis in Miami Lakes, FL. It is covered by poly-n-butyl methacrylate and releases the drug over 30 days [21].
Complications
Too much antiproliferation can retard or inhibit arterial wall healing, which leads to chronic inflammation [9]. One study proved that arterial wall healing was incomplete at 180 days with drug eluting stents, where as complete healing was seen with bare metal stents after 28 days [22]. In 2006, the European Society of Cardiology Congress cited stents releasing paclitaxel or sirolimus as slightly increasing the risk of thrombosis [9,16]. Although the occurence of stent thrombosis is rare, the absolute occurence using DES's was nearly double that of BMS's (1.2% Sirolimus and 1.3% Paclitaxel vs. 0.6% Bare Metal Stents) [23,24]. As of today, clinical studies of paclitaxel-eluting stents have ceased, and sirolimus-eluting stents are no longer manufactured [16].
Second Generation Drug Eluting Stents
New generation stents release everolimus and zotarolimus [9]. These stents are promising in that they reduce the risk of thrombosis, one of the drawbacks of their first generation counterparts. Clinical trials have demonstrated that everolimus reduces the risk of repeat revascularization, heart attack, and stent thrombosis compared to paclitaxel and sirolimus eluting stents [9]. Compared with paclitaxel, zotarolimus reduces the risk of myocardial infraction [9].
Zotarolimus is a derivative of sirolimus. Medtronic has developed the Endeavor zotarolimus-eluting stent, which is constructed from cobalt-chrome, coated with phorlycholine, and elutes the drug over 2 weeks [16]. Clinical trials have demonstrated a reduced risk of thrombosis of 0.5%, however it has not yet been proven that the zotarolimus eluting stent is superior to first generation DES's with regards to safety [26]
Everolimus is similar to sirolimus, but is more lipophilic and therefore able to diffuse more rapidly into the arterial wall. Evrolimus-eluting stents constructed from L605 cobalt-chromium and coated with poly-n-butyl methacrylate are manufactured by Abbott Laboratories and Promus [27]. The Xience prime is available in sizes of 2.25 - 4.0 mm and up to 38 mm long. These stents exhibit superior long term safety traits in comaprison to Paclitaxel-eluting stents , including reduced thrombosis, myocardial infraction, and revascularization [28].
Studies have suggested that stent strut size and alloys affect endothelial cell recovery, which prompted the development of cobalt-chrome and platinum-chrome stents. These stents exhibit improved radial strength with thinner struts of 80-90μm [18]. Thinner struts also cause less arterial injury, thus reducing thrombosis and restenosis and speeding arterial wall healing [9]. Second generation stents are being engineered using these platforms.
Fully resorbable biodegradable stents (BRS) have entered clinical trials, and the first implantation of the poly-L-lactic acid Igaki-Tamai BRS in a human has been performed [9,29]. Preclinical evaluation of the Abbott everolimus eluting ABSORB stent is currently underway [29]. However, it it yet to be determined if these devices can meet or exceed the safety and efficacy of available stents [9].
Endothelialization and Restenosis
Endothelialization is the natural growth of endothelial cells and tissue which occurs rapidly after the implantation of a coronary stent. Stents cannot be removed due to Endothelialization of the arterial wall. Endothelialization after stent placement can often lead to stent thrombosis due to negative remodeling and restenosis. Thrombosis was originally the most important concern immediately following angioplasty, formation of a thrombus and occlusion in a stent treated artery can lead to myocardial infarction and death. Luckily anti-platelet drugs such as aspirin and clopidogrel (Plavix) along with the use of antiproliferative drugs in drug eluting stents, have reduced the risk of post-stent thrombosis dramatically. The use of antiproliferative drugs in drug eluting stents has been controversial. Too much anti-proliferative presence can prevent the reformation of a functional endothelial layer which may leave it even more vulnerable to restenosis then without the drug present originally [30,31,32]
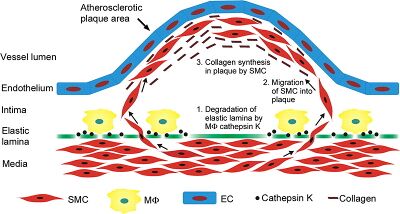
Restenosis is characterized by proliferation of neointima (neointimal hyperplasia). Neointima is scar tissue within an artery that forms as a result of angioplasty or stent placement due to proliferation of smooth muscle cells. Neointima forms by a system of protein interactions in which extra cellular matrix glycoproteins inhibit smooth muscle cells from adhering to the extra cellular matrix, allowing them to migrate from the media to the intima. This is catalyzed by macrophages that degrade the elastic lamina allowing SMC migration. This results in the reformation of atherosclerotic lesions. The smooth muscle cells divide and produce collagen inside the intima, forcing the upward bulge of the lesion/plaque, which further attracts plaque eventually leading to thrombosis. This also leads to increased arterial pressure due to smooth muscle contractile forces, increased arterial wall shear stress, and decreased blood flow through the artery. Restenosis is often characterized by very serious angina (more so than original occlusion) [30,31,32].
Gene Eluting Stents
Current research in humans has focused on the use of bio absorbable and bioresorbable materials in stents. The use of Poly L. Lactic acid has been the most promising, although magnesium has shown to be a metal bio absorbable alternative to polymers. The main risks associated with bioresorbable stents include the stents dissolving too quickly for adequate vessel healing, and not providing adequate support/scaffolding leading to detach from arterial wall [5].
Other research has focused on using nanoparticle based drug delivery systems, that better target individual proteins inside cells, as well as nanofibrous scaffolds that mimic ECM to facilitate Endothelialization and maximize anti proliferation of SMCs. The use of combination drug eluting stents is also being explored [5].
Gene eluting stents have been developed in animal models (pigs, rabbits) that release targeted gene therapy through a polymer or nanoparticle based delivery system. These GES have used nonviral and viral gene vectors, although non-viral seem to work better. RNA interference, Anti Sense DNA, and plasmid DNA have been used in combination with lipid and polymer based nanoparticles. This gives specificity and ability to directly target bioprocess of interest. So far over 30 genes related to four main processes have emerged as potential targets. The four processes of interest are generally Endothelialization, SMC proliferation, Thrombosis, and inflammation. Combination drug and gene therapy is also being researched. The general goal of gene therapy and combination drug/gene therapy is to increase smooth Endothelialization, minimize SMC proliferation, reduce growth of neointima, and reduce inflammation and thrombosis [5]. It has also been shown that stainless steel stents seeded with mesenchymal stem cells can reduce SMC proliferation and facilitate Endothelialization having an anti-restenotic effect [33].
Animal Models
Stent testing in animals has revealed that upon placement of a bare metal steel stent in coronary arteries (healthy or nonhealthy), the animals exhibit the same injury response as humans when the same stents are implanted: neointimal formation and endothelialization [38]. The reason animal testing is desired is because human healing is 5-6 times longer than that of a pig or rabbit, meaning more progress could be made by testing stents on animals [38].
A wide variety of animals have been used for atherosclerosis and thrombosis studies including: primary rodents, rabbits, nonhuman primates, and dogs. While rodents, primarily mice, are low cost, easy to handle, and readily available, they are unable to develop atherosclerosis unless a genetic mutation is induced, making them unideal to test stents on and thus unrepresentative of how humans would respond to stents [29]. Nonhuman primates exhibit very similar physical properties and responses to that of humans, and would be ideal to test stents on but it is highly costly to do so, therefore they are not the most readily used model. Dogs do have similar physical properties of humans, but do not readily undergo atherosclerosis, even under specific dietary conditions and therefore are not ideal to test stents on. Pigs, however, spontaneously develop atherosclerosis under dietary conditions, and have similar physical properties as humans and are therefore considered an ideal candidate for stent testing [39].
One study conducted tested drug eluting stents on pigs and rabbit coronary arteries to test the injury response and overall healing timeline. Sirolimus drug eluting stents were tested on only pigs and paclitaxel drug eluting stents were tested on both pigs and rabbits over a period up to 180 days, with checkups on day 14, 28, 90, and 180. It was found that by the 90th day all of the drug had been released prior, and that by the 180th day healing was completed. Researchers believe that since animals were able to heal by this point, that it gives an estimate that in humans healing should be completed in an approximate time period of 2-3 years [28]. However, animals the stents are tested on are usually healthy and do not likely experience the coronary artery disease that humans do, so this could be one factor as to why the animals heal so quickly [38].
Current Research
Overlapping Bare Metal Stents
While stents are traditionally used to prevent the collapsing of vessels, current research is being applied to stabilizing aneurysms. Overlapping bare-metal stents are placed at the cite of an aneurysm in order to strengthen the weakened vessel by reducing the blood flow through the aneurysm. This controlled flow decreases stress on the vessel walls, and promotes platelet growth. As the vessel continues to strengthen, the aneurysm degrades. Despite the high success rate of current findings, overlapping bare-metal stents still have significant development. Current obstacles faced in this area of research include varying blood flow and anatomical differences among patients [40].
Stents Loaded with Growth Factors
Recent studies have used stents coated with hyaluronic acid, heparin, and two growth factors to trigger the rapid and tight re-endothelialization of the injured vessel being utilized [40]. The growth factors used were heparin-binding domains. The reason this is important is that heparin-binding surfaces can be applied to mettalic biomaterial surfaces, meaning that they could be applied to stents to help reduce the natural reaction normally exhibited by vessels when using other metallic biomaterial treated surfaces [40].
History
3000 B.C. - Metal pipes are used by the Egyptians in bladder catheterizations [34]
400 B.C. - Hollow reeds and pipes are used as catheters for studying heart valves in cadavers [34]
1711 - The first cardiac catheterization of a horse is conducted by Hales using brass pipes, a glass tube, and a goose trachea [34]
1929 - German Dr. Werner Forssmann performs first human cardiac catheterization [34]
1958 - The diagnostic coronary angiogram is developed by Dr. Mason Sones [34]
1964 - Doctor Charles Dotter introduces transluminal angioplasty, or the concept of remodeling the artery [34]
1974 - First peripheral human balloon angioplasty performed by Andreas Gruentzig [34]
1977 - First Percutaneous coronary intervention was performed Gruentzig [9]
1980 - 1000 angioplasties are performed worldwide, and guiding catheters are developed [34]
1982 - Coaxial balloon systems, brachial guiding catheters, and steerable guide wires are developed
1986 - Puel and Sigwart deployed the first coronary stent to act as a scaffold to prevent vessel closure during PTCA and vessel closure [35]
1994 - 250000 PTCA's performed each year, and the Palmaz-Schatz stent gains F.D.A. approval in the US [35,34]
1999 - Stenting associated with 84.2% of all PCI's [35]
2001 - 2 million angioplasties performed worldwide, and the introduction of drug eluting stents [15]
2003 - Sirolimus eluting stent, the Cypher, is introduced [34,37]
2004 - Paclitaxel eluting stent, the Taxus, is introduced [25,34]
2007 - 90% of stents implanted in US and Europe were drug eluting [15]
2012 - Over 500,000 patients implanted with stents in the US per year [9]
Sources
[1] "cardiovascular disease." Encyclopædia Britannica. Encyclopædia Britannica Online Academic Edition. Encyclopædia Britannica Inc., 2013. Web. 28 Feb. 2013. <http://www.britannica.com/EBchecked/topic/720793/cardiovascular-disease>.
[2] https://www.nhlbi.nih.gov/health/health-topics/topics/atherosclerosis
[3] http://umm.edu/health/medical/altmed/condition/atherosclerosis
[4] Roberts R, Stewart AR. Genes and Coronary Artery Disease: Where Are We?. J Am Coll Cardiol. 2012;60(18):1715-1721. doi:10.1016/j.jacc.2011.12.062
[5] (13) Yin, Rui-Xing, De-Zhai Yang, and Jin-Zhen Wu. "Nanoparticle Drug-and Gene-eluting Stents for the Prevention and Treatment of Coronary Restenosis." Theranostics 4.2 (2014): 175.
[6] Basic Science for Clinicians: Genetic Basis of Atherosclerosis: Part I: New Genes and Pathways Aldons J. Lusis, Alan M. Fogelman, and Gregg C. Fonarow Circulation. 2004;110:1868-1873, doi:10.1161/01.CIR.0000143041.58692.CC
[7] http://www.radiologyinfo.org/en/info.cfm?pg=angioplasty
[8] Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356: 1503–1516.
[9] Stefanini G., Holmes D. Drug eluting coronary-artery stents. New Engl J Med 2013;368:254-65.
[10] Intracoronary Stenting: From Concept to Custom Peter N. Ruygrok and Patrick W. Serruys Circulation. 1996;94:882-890, doi:10.1161/01.CIR.94.5.882.
[11] Caramori PRA, Lima VC, Seidelin PH, et al. Long-term endothelial dysfunction after coronary stenting. J Am Coll Cardiol1999;34:1675-9.
[12] McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004;364:1519-21.
[13] Moliterno DJ. Healing Achilles—sirolimus versus paclitaxel. N Engl J Med 2005;353:724-6.
[14] usatoday30.usatoday.com
[15] Biondi-Zoccai GG, Agostoni P, Abbate A, et al. Adjusted indirect comparison of intracoronary drug-eluting stents: evidence from a metaanalysis of randomized bare-metal-stent-controlled trials. Int J Cardiol2005;100:119-23.
[16] Townsend JC, Rideout P, Steinberg DH. Everolimus-eluting stents in interventional cardiology.
[17] Lagerqvist, Bo, Stefan K. James, Ulf Stenestrand, Johan Lindbäck, Tage Nilsson, and Lars Wallentin. “Long-Term Outcomes with Drug-Eluting Stents versus Bare-Metal Stents in Sweden.” New England Journal of Medicine 356, no. 10 (March 8, 2007): 1009–1019. doi:10.1056/NEJMoa067722.
[18] Chitkara K, Pujara K. Drug-eluting stents in acute coronary syndrome: is there a risk of stent thrombosis with second-generation stents? Eur J Cardiovasc Med. 2010;1:20–24.
[19] Axel DI, Kunert W, Goggelmann C, et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645.
[20] Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo). 1975;28:721–726.
[21] Sheiban I, Villata G, Bollati M, Sillano D, Lotrionte M, Biondi-Zoccai G. Next-generation drug-eluting stents in coronary artery disease: Focus on everolimus-eluting stent (Xience V). Vasc Health Risk Manag. 2008;4:31–38.
[22] Tsimikas S. Drug-eluting stents and late adverse clinical outcomes: lessons learned, lessons awaited. J Am Coll Cardiol 2006;47:2112-5.
[23] Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet. 2007;369:667–678.
[24] Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029.
[25] Stone, Gregg W., Jeffrey W. Moses, Stephen G. Ellis, Joachim Schofer, Keith D. Dawkins, Marie-Claude Morice, Antonio Colombo, et al. “Safety and Efficacy of Sirolimus- and Paclitaxel-Eluting Coronary Stents.” New England Journal of Medicine 356, no. 10 (March 8, 2007): 998–1008. doi:10.1056/NEJMoa067193.
[26] Fajadet J, Wijns W, Laarman GJ, et al. Randomized, 1lind, multicenter study of the endeavor zotarolimus-eluting phosphorylcholine-encapsulated stent for treatment of native coronary artery lesions: Clinical and angiographic results of the ENDEAVOR II trial. Circulation. 2006;114:798–806.
[27] Weisz G, Leon MB, Holmes DR Jr, et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (sirolimus-eluting stent in de-novo native coronary lesions) trial. J Am Coll Cardiol. 2009;53:1488–1497.
[28] Smits PC, Kedhi E, Royaards KJ, et al. 2-year follow-up of a randomized controlled trial of everolimus- and paclitaxel-eluting stents for coronary revascularization in daily practice. COMPARE (Comparison Of The Everolimus Eluting Xience-V Stent With The Paclitaxel Eluting Taxus Liberte stent in all-comers: a randomized open label trial). J Am Coll Cardiol. 2011;58:11–18.
[29] Bourantas CV, Onuma Y, Farooq V, Zhang Y, Garcia H, Serruys P. Bioresorbable scaffolds: Current knowledge, potentialities and limitations experienced during their first clinical applications. Int J Cardiology. 2012, Article in Press.
[30] Zargham R (2008) Preventing restenosis after angioplasty: a multistage approach. Clin Sci (Lond) 114: 257–264. [PubMed].
[31] Neointima formation after acute vascular injury. Role of counteradhesive extracellular matrix proteins. M W Majesky. Tex Heart Inst J. 1994; 21(1): 78–85. PMCID: PMC325135.
[32] Mechanisms of restenosis after coronary intervention: difference between plain old balloon angioplasty and stenting. Masaki Nakatani, Youichi Takeyama, Masayuki Shibata, Minoru Yorozuya, Hiroshi Suzuki, Shinji Koba, Takashi Katagiri Cardiovasc Pathol. 2003 Jan-Feb; 12(1): 40–48.
[33] Wu, X., Wang, G., Tang, C., Zhang, D., Li, Z., Du, D. and Zhang, Z. (2011), Mesenchymal stem cell seeding promotes reendothelialization of the endovascular stent. J. Biomed. Mater. Res., 98A: 442–449. doi: 10.1002/jbm.a.33133.
[34] Mueller R, Sanborn T.The history of interventional cardiology. Am Heart J. 1995;129:146-72.
[35] Serruys PW, Kutryk MJB, Ong ATL. Coronary-artery stents. N Engl J Med 2006;354:483-95.
[36] http://www.ptca.org
[37] Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323.
[38] Virmani, R.; Kolodgie, F. D.; Farb, A.; Lafont, A. Drug Eluting Stents: Are Human and Animal Studies Comparable? Heart. 2003, 89, 133–138.
[39] Vilahur, G.; Pedro, T.; Badimon, L. Atherosclerosis And Thrombosis: Insights from Large Animal Models. Journal of Biomedicine and Biotechnology. 2011, 2011, 1–12.
[40] Zhang, P.; Liu, X.; Sun, A.; Fan, Y.; Deng, X. Hemodynamic Insight Into Overlapping Bare-Metal Stents Strategy in the Treatment of Aortic Aneurysm. Journal of Biomechanics. 2015, 48, 2041–2046.
[41] Choi, D. H.; Kang, S. N.; Kim, S. M.; Gobaa, S. Growth Factors-Loaded Stents Modified with Hyaluronic Acid and Heparin for Induction of Rapid and Tight Re-Endothelialization. Colloids and Surfaces B: Biointerfaces. 2016, 141, 602–610.