BIO254:Silent: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
==Introduction== | ==Introduction== | ||
A '''silent synapse''' is a special type of excitatory glutamatergic synapse that relies on NMDA receptors to the exclusion of AMPA receptors. The voltage dependency of NMDA receptors causes them to act as logical AND gates, requiring both postsynaptic depolarization and glutamate binding to trigger an excitatory postsynaptic potential (EPSP). | A '''silent synapse''' is a special type of excitatory glutamatergic synapse that relies on NMDA receptors to the exclusion of AMPA receptors. The voltage dependency of NMDA receptors causes them to act as logical "AND" gates, requiring both postsynaptic depolarization and glutamate binding to trigger an excitatory postsynaptic potential (EPSP). | ||
==Silent Synapses== | ==Silent Synapses== | ||
Several experiments demonstrate that excitatory synapses can effectively regulate their postsynaptic glutamate receptors. For instance, when some glutamatergic synapses are | Several experiments demonstrate that excitatory synapses can effectively regulate their postsynaptic glutamate receptors. For instance, when some glutamatergic synapses are stimulated, no postsynaptic electrical signal is generated when the postsynaptic cell is at a normal resting membrane potential. In contrast, when these same postsynaptic cells are depolarized, these "silent synapses" are able to transmit strong postsynaptic responses that are detectable using electrophysiological methods (such as patch clamp). Because these silent synapses have the potential to be turned on or off in response to postsynaptic activity, this mechanism demonstrates a simple means for modifying and regulating neural activity. | ||
Silent synapses are abundant in development and are found in several brain regions, including the hippocampus, the cerebral cortex, and the spinal cord. The "silence" of these synapses is the result of Mg++ blockade of NMDA receptors, which are voltage-dependent. Interestingly, glutamate released at silent synapses | Silent synapses are abundant in development and are found in several brain regions, including the hippocampus, the cerebral cortex, and the spinal cord. The "silence" of these synapses is the result of Mg++ blockade of NMDA receptors, which are voltage-dependent. Interestingly, glutamate released at silent synapses binds only to NMDA receptors, without binding to AMPA receptors. For years, this specificity has puzzles neurobiologists, but one explanation is that NMDA and AMPA receptors have significantly different affinities for binding the released glutamate neurotransmitter. Glutamate released onto neighboring neurons may diffuse only to synapses on the neuron from which the electrical recording is being taken. The concentration of glutamate may be sufficient enough to activate NMDA receptors (high-affinity), but not the low-affinity AMPA receptors. A second possibility states that both AMPA and NMDA receptors exist on the postsynaptic terminal, but only the NMDA receptors are fully functional. Or, some specific excitatory synapses only have NMDA receptors; growing evidence tends to support this latter model. Immunocytochemical experiments perhaps provide the most compelling evidence for this explanation: staining done by Gomperts et al. (2000) shows that select excitatory synapses only possess NMDA receptors. | ||
The abundance of NMDA-receptor-only synapses peaks after post-natal development and decreases in adults. Hence, silent synapses appear not to be a separate class of excitatory synapses that are deficient in AMPA receptors, but are developmentally | The abundance of NMDA-receptor-only synapses peaks soon after post-natal development and decreases in adults. Hence, silent synapses appear not to be a separate class of excitatory synapses that are deficient in AMPA receptors, but are seen developmentally at an early stage of glutamatergic synapse maturation. | ||
==The iGluR channel== | ==The iGluR channel== | ||
Most excitatory synapses in the central nervous system are glutamatergic. In these synapses, glutamate released by the presynaptic cell acts on both metabotropic (mGluR) and ionotropic | Most excitatory synapses in the central nervous system are glutamatergic. In these synapses, glutamate released by the presynaptic cell acts on both metabotropic (mGluR) and ionotropic (iGluR) glutamate receptors in the postsynaptic membrane. Receptors in the iGluR channel can be classified as either NMDA (N-methyl-D-aspartate) or non-NMDA (kainate and AMPA) receptors. | ||
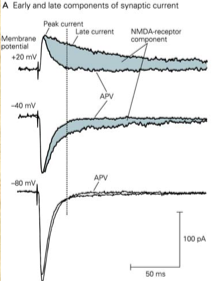
Non-NMDA receptors contribute to the early phase of the excitatory postsynaptic current (EPSC) and generate peak current, whereas NMDA receptors contribute to the late phase as a slower component, as can be seen in the image below. This image also shows the effect of APV (see next section) on the EPSC: | Non-NMDA receptors contribute to the early phase of the excitatory postsynaptic current (EPSC) and generate peak current, whereas NMDA receptors contribute to the late phase as a slower component, as can be seen in the image below. This image also shows the effect of R-2-amino-5-phosphonopentanoate (APV), an inhibitor of NMDA receptors (see next section), on the EPSC: | ||
<div style="margin-left: 60px;">[[Image:iGluR_phases.png]]</div> | <div style="margin-left: 60px;">[[Image:iGluR_phases.png]]</div> | ||
| Line 24: | Line 24: | ||
NMDA receptors additionally require postsynaptic depolarization to eject a Mg<sup>2+</sup> ion that blocks the channel during normal operation. As a result, the relative contribution of NMDA receptors to the EPSC depends on the postsynaptic membrane potential. | NMDA receptors additionally require postsynaptic depolarization to eject a Mg<sup>2+</sup> ion that blocks the channel during normal operation. As a result, the relative contribution of NMDA receptors to the EPSC depends on the postsynaptic membrane potential. | ||
Also unlike AMPA receptors, open NMDA receptors permit the influx of Ca<sup>2+</sup>, which plays a role in LTP (see below). | Also unlike AMPA receptors, open NMDA receptors permit the influx of Ca<sup>2+</sup>, which plays a role in long-term potentiation (LTP) (see below). | ||
The inactivity of an NMDA-only synapse when the postsynaptic cell is polarized below -40 mV gives the '''silent synapse''' its name. | The inactivity of an NMDA-only synapse when the postsynaptic cell is polarized below -40 mV gives the '''silent synapse''' its name. | ||
NMDA receptors are actively inhibited by APV | NMDA receptors are actively inhibited by APV, which can thereby regulate '''silent synapse''' activity. | ||
==Molecular Mechanisms Underlying LTP== | ==Molecular Mechanisms Underlying LTP== | ||
Although LTP was discovered more than three decades ago, the molecular mechanisms contributing to this phenomenon are still not well understood. The properties of NMDA-type glutamate receptors were first elucidated in the mid-1980s, and at about the same time, neurobiologists found that antagonists (inhibitors) of NMDA receptors actually prevented LTP. The " | Although LTP was discovered more than three decades ago, the molecular mechanisms contributing to this phenomenon are still not well understood. The properties of NMDA-type glutamate receptors were first elucidated in the mid-1980s, and at about the same time, neurobiologists found that antagonists (inhibitors) of NMDA receptors actually prevented LTP. The "AND" characteristics of NMDA receptors contribute to both the specificity and associativity of LTP. For example, when only one group of synaptic inputs is strongly stimulated, LTP is confined to the active synapses (selectivity), since glutamate opens NMDA receptors only at the stimulated sites. However, in terms of associativity, applying a weakly stimulating input current releases glutamate but cannot depolarize the post-synaptic terminal enough to relieve the Mg++ block. When neighboring stimulations are applied to a weak input, these currents work "associatively" to both depolarize and unblock the NMDA receptors on the cell dendrite. | ||
==Long-term potentiation== | ==Long-term potentiation (LTP)== | ||
[[BIO254:LTP|Long-term potentiation]] (LTP) describes the process wherein the synaptic efficacy of two neurons is strengthened over time, in a way that depends on the simultaneity of firing (spike timing dependent plasticity). The best-studied form of this is hippocampal CA3-CA1 LTP, demonstrated by Timothy Bliss and Terje Lomo (1973). Brief high-frequency (tetanic) stimulation of a presynaptic cell can result in long-term enhancement of synaptic transmission. LTP additionally exhibits the following properties: | [[BIO254:LTP|Long-term potentiation]] (LTP) describes the process wherein the synaptic efficacy of two neurons is strengthened over time, in a way that depends on the simultaneity of firing (spike timing-dependent plasticity). The best-studied form of this is hippocampal CA3-CA1 LTP, demonstrated by Timothy Bliss and Terje Lomo (1973). Brief high-frequency (tetanic) stimulation of a presynaptic cell can result in long-term enhancement of synaptic transmission. LTP additionally exhibits the following properties: | ||
* '''Cooperativity''': The probability of inducing LTP increases with the number of stimulated afferents, and the strength of their stimulation. This reflects the postsynaptic depolarization threshold that must be exceeded in order to induce LTP. The voltage dependency of NMDAR establishes this threshold. | * '''Cooperativity''': The probability of inducing LTP increases with the number of stimulated afferents, and the strength of their stimulation. This reflects the postsynaptic depolarization threshold that must be exceeded in order to induce LTP. The voltage dependency of the NMDA receptor (NMDAR) establishes this threshold. | ||
* '''Input specificity''': LTP is restricted to the synapses that triggered the process, and does not propagate to nearby synapses. | * '''Input specificity''': LTP is restricted to the synapses that triggered the process, and does not propagate to nearby synapses. | ||
* '''Associativity''': Weak stimulation | * '''Associativity''': Weak stimulation of one pathway may be insufficient to induce LTP, though when coupled with strong stimulation of another, LTP can be induced in both pathways. | ||
It was found that CA3-CA1 LTP requires both NMDAR and Ca<sup>2+</sup>, and involves depolarization of the postsynaptic cell, activation of NMDA receptors in that cell, the resulting influx of Ca<sup>2+</sup>, and the activation of secondary messengers by Ca<sup>2+</sup>. | It was found that CA3-CA1 LTP requires both NMDAR and Ca<sup>2+</sup>, and involves depolarization of the postsynaptic cell, activation of NMDA receptors in that cell, the resulting influx of Ca<sup>2+</sup>, and the activation of secondary messengers by Ca<sup>2+</sup>. | ||
Revision as of 19:54, 23 October 2006
Introduction
A silent synapse is a special type of excitatory glutamatergic synapse that relies on NMDA receptors to the exclusion of AMPA receptors. The voltage dependency of NMDA receptors causes them to act as logical "AND" gates, requiring both postsynaptic depolarization and glutamate binding to trigger an excitatory postsynaptic potential (EPSP).
Silent Synapses
Several experiments demonstrate that excitatory synapses can effectively regulate their postsynaptic glutamate receptors. For instance, when some glutamatergic synapses are stimulated, no postsynaptic electrical signal is generated when the postsynaptic cell is at a normal resting membrane potential. In contrast, when these same postsynaptic cells are depolarized, these "silent synapses" are able to transmit strong postsynaptic responses that are detectable using electrophysiological methods (such as patch clamp). Because these silent synapses have the potential to be turned on or off in response to postsynaptic activity, this mechanism demonstrates a simple means for modifying and regulating neural activity.
Silent synapses are abundant in development and are found in several brain regions, including the hippocampus, the cerebral cortex, and the spinal cord. The "silence" of these synapses is the result of Mg++ blockade of NMDA receptors, which are voltage-dependent. Interestingly, glutamate released at silent synapses binds only to NMDA receptors, without binding to AMPA receptors. For years, this specificity has puzzles neurobiologists, but one explanation is that NMDA and AMPA receptors have significantly different affinities for binding the released glutamate neurotransmitter. Glutamate released onto neighboring neurons may diffuse only to synapses on the neuron from which the electrical recording is being taken. The concentration of glutamate may be sufficient enough to activate NMDA receptors (high-affinity), but not the low-affinity AMPA receptors. A second possibility states that both AMPA and NMDA receptors exist on the postsynaptic terminal, but only the NMDA receptors are fully functional. Or, some specific excitatory synapses only have NMDA receptors; growing evidence tends to support this latter model. Immunocytochemical experiments perhaps provide the most compelling evidence for this explanation: staining done by Gomperts et al. (2000) shows that select excitatory synapses only possess NMDA receptors.
The abundance of NMDA-receptor-only synapses peaks soon after post-natal development and decreases in adults. Hence, silent synapses appear not to be a separate class of excitatory synapses that are deficient in AMPA receptors, but are seen developmentally at an early stage of glutamatergic synapse maturation.
The iGluR channel
Most excitatory synapses in the central nervous system are glutamatergic. In these synapses, glutamate released by the presynaptic cell acts on both metabotropic (mGluR) and ionotropic (iGluR) glutamate receptors in the postsynaptic membrane. Receptors in the iGluR channel can be classified as either NMDA (N-methyl-D-aspartate) or non-NMDA (kainate and AMPA) receptors.
Non-NMDA receptors contribute to the early phase of the excitatory postsynaptic current (EPSC) and generate peak current, whereas NMDA receptors contribute to the late phase as a slower component, as can be seen in the image below. This image also shows the effect of R-2-amino-5-phosphonopentanoate (APV), an inhibitor of NMDA receptors (see next section), on the EPSC:
NMDA receptors (NMDAR)
NMDA receptors additionally require postsynaptic depolarization to eject a Mg2+ ion that blocks the channel during normal operation. As a result, the relative contribution of NMDA receptors to the EPSC depends on the postsynaptic membrane potential.
Also unlike AMPA receptors, open NMDA receptors permit the influx of Ca2+, which plays a role in long-term potentiation (LTP) (see below).
The inactivity of an NMDA-only synapse when the postsynaptic cell is polarized below -40 mV gives the silent synapse its name.
NMDA receptors are actively inhibited by APV, which can thereby regulate silent synapse activity.
Molecular Mechanisms Underlying LTP
Although LTP was discovered more than three decades ago, the molecular mechanisms contributing to this phenomenon are still not well understood. The properties of NMDA-type glutamate receptors were first elucidated in the mid-1980s, and at about the same time, neurobiologists found that antagonists (inhibitors) of NMDA receptors actually prevented LTP. The "AND" characteristics of NMDA receptors contribute to both the specificity and associativity of LTP. For example, when only one group of synaptic inputs is strongly stimulated, LTP is confined to the active synapses (selectivity), since glutamate opens NMDA receptors only at the stimulated sites. However, in terms of associativity, applying a weakly stimulating input current releases glutamate but cannot depolarize the post-synaptic terminal enough to relieve the Mg++ block. When neighboring stimulations are applied to a weak input, these currents work "associatively" to both depolarize and unblock the NMDA receptors on the cell dendrite.
Long-term potentiation (LTP)
Long-term potentiation (LTP) describes the process wherein the synaptic efficacy of two neurons is strengthened over time, in a way that depends on the simultaneity of firing (spike timing-dependent plasticity). The best-studied form of this is hippocampal CA3-CA1 LTP, demonstrated by Timothy Bliss and Terje Lomo (1973). Brief high-frequency (tetanic) stimulation of a presynaptic cell can result in long-term enhancement of synaptic transmission. LTP additionally exhibits the following properties:
- Cooperativity: The probability of inducing LTP increases with the number of stimulated afferents, and the strength of their stimulation. This reflects the postsynaptic depolarization threshold that must be exceeded in order to induce LTP. The voltage dependency of the NMDA receptor (NMDAR) establishes this threshold.
- Input specificity: LTP is restricted to the synapses that triggered the process, and does not propagate to nearby synapses.
- Associativity: Weak stimulation of one pathway may be insufficient to induce LTP, though when coupled with strong stimulation of another, LTP can be induced in both pathways.
It was found that CA3-CA1 LTP requires both NMDAR and Ca2+, and involves depolarization of the postsynaptic cell, activation of NMDA receptors in that cell, the resulting influx of Ca2+, and the activation of secondary messengers by Ca2+.
The specific expression mechanisms of CA3-CA1 LTP are highly controversial. However, we do know that the expression of LTP is likely to involve both pre- and postsynaptic mechanisms, and that the probability of presynaptic neurotransmitter release is increased after LTP induction. At the postsynaptic cell, AMPA receptors are inserted into the cell membrane, which increases the conductance of the AMPA channel and thereby converts silent synapses into functional ones.
After the early phase of LTP (E-LTP) in which these pre- and post-synaptic changes occur, the late phase (L-LTP) can lead to the formation of new synapses.
Unlike CA3-CA1 LTP, mossy fiber LTP is not dependent on NMDAR, and might be expressed primarily by increased presynaptic neurotransmitter release.
Recent updates to the site
List of abbreviations:
- N
- This edit created a new page (also see list of new pages)
- m
- This is a minor edit
- b
- This edit was performed by a bot
- (±123)
- The page size changed by this number of bytes
4 February 2026
| 21:31 | CHIP:Talks diffhist +543 Gabor Balazsi talk contribs | ||||
|
|
18:11 | Altman:Pictures 2 changes history +103 [David Altman (2×)] | |||
|
|
18:11 (cur | prev) +1 David Altman talk contribs | ||||
|
|
18:11 (cur | prev) +102 David Altman talk contribs | ||||
|
|
18:09 | (Upload log) [David Altman (11×)] | |||
|
|
18:09 David Altman talk contribs uploaded File:Makingaflowcell.jpeg | ||||
|
|
18:08 David Altman talk contribs uploaded File:MitchSeptoffpic2.jpeg | ||||
|
|
18:06 David Altman talk contribs uploaded File:MitchSeptoffpic.jpeg | ||||
|
|
18:05 David Altman talk contribs uploaded File:MitchSeptoffpic.jpg | ||||
|
|
17:45 David Altman talk contribs uploaded File:Myosincartoonv2.jpg | ||||
|
|
17:43 David Altman talk contribs uploaded a new version of File:Myosincartoon.jpg | ||||
|
|
17:36 David Altman talk contribs uploaded File:Journal.pcbi.1000694.s014 AMFvkY.gif | ||||
|
|
07:16 David Altman talk contribs uploaded File:Myosincartoon.jpg | ||||
|
|
07:09 David Altman talk contribs uploaded File:GoodsellKinesin.jpg | ||||
|
|
06:37 David Altman talk contribs uploaded File:KinesinWalkingv2.gif | ||||
|
|
06:28 David Altman talk contribs uploaded File:KinesinWalking.gif | ||||
|
|
18:08 | Altman:Lab Members 3 changes history +8 [David Altman (3×)] | |||
|
|
18:08 (cur | prev) +1 David Altman talk contribs (→Current Lab Members) | ||||
|
|
18:06 (cur | prev) +1 David Altman talk contribs (→Current Lab Members) | ||||
|
|
18:05 (cur | prev) +6 David Altman talk contribs (→Current Lab Members) | ||||
| 18:01 | Altman:WUbites diffhist −9 David Altman talk contribs (→WUbites) | ||||