BISC110/F12: Series 3 Experiment 9 Hill Reaction: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| Line 109: | Line 109: | ||
#Use 100% Adjust Knob (B) to set meter to 100%T, which is equivalent to zero absorbance on the absorbance scale. Remove the blank tube and save it in your ice bucket. | #Use 100% Adjust Knob (B) to set meter to 100%T, which is equivalent to zero absorbance on the absorbance scale. Remove the blank tube and save it in your ice bucket. | ||
<br><br> | <br><br> | ||
'''Varying the Reaction Rate:''' The Hill reaction occurs at different rates depending on the light intensity and the quality and concentration of the thylakoids. Our goal today is to determine the best light intensity and thylakoid concentration for a 90 sec. reaction. You will measure the reaction rates by taking absorbance readings over that time period in 15 s intervals. We will use the drop in absorbance over time as a measure of reduction of blue DCPIP to colorless DCPIPH. If we can control all the variables properly, this reduction rate should also be | '''Varying the Reaction Rate:''' The Hill reaction occurs at different rates depending on the light intensity and the quality and concentration of the thylakoids. Our goal today is to determine the best light intensity and thylakoid concentration for a 90 sec. reaction. You will measure the reaction rates by taking absorbance readings over that time period in 15 s intervals. We will use the drop in absorbance over time as a measure of reduction of blue DCPIP to colorless DCPIPH. If we can control all the variables properly, this reduction rate should also be an indication of the rate of photosynthesis since the light and dark reactions are coupled. <br><br> | ||
Depending on the number, distribution (clumped or not) and condition of thylakoids in your reaction tube, you may obtain varying reaction rates with a given light intensity. Therefore, you will test your thylakoids neat (undiluted) and diluted 1/2 with resuspension solution. We measure the incident light using a light meter in units of µmol photons m-2 s-1. <br><br> | Depending on the number, distribution (clumped or not) and condition of thylakoids in your reaction tube, you may obtain varying reaction rates with a given light intensity. Therefore, you will test your thylakoids neat (undiluted) and diluted 1/2 with resuspension solution. We measure the incident light using a light meter in units of µmol photons m-2 s-1. <br><br> | ||
Typically, the light intensity is set at ~80 µmol photons m-2 s-1 in order to drive the reaction at the desired rate—a drop in absorbance per 15 s that is consistent throughout a 90 s trial. However, that intensity may not be appropriate for all thylakoid preparations at all concentrations so you will have to experiment with both the light intensity and the concentration of your thylakoid suspension to find a photosynthetic rate that does not use up all of our substrate (DCPIP) too quickly (before 90 sec.) or that is too slow to approach substrate depletion in 120 seconds. The 15 s reading intervals consists of 10 s of thylakoid illumination and 5 s to read the absorbance in the spectrophotometer. Your goal is to establish conditions favorable for a 90 s experiment. This data collection at interval of 15 s should yield 7 data points (i.e., absorbance readings at time = 0, 15, 30, 45, 60, 75, and 90 s) that are all in the linear portion of a curve when you graph absorbance (y axis) vs. time (x axis). If your light intensity is too high and/or your thylakoids too concentrated, your curve will flatten before the 90 second reading because you have depleted your DCPIP substrate and those data points after substrate depletion will be unusable. If your light intensity is too low and/or your thylakoids too dilute or of poor quality, the data points obtained within an optimal 90 sec. reaction will not measure enough of the linear part of the reaction. <br><br> | Typically, the light intensity is set at ~80 µmol photons m-2 s-1 in order to drive the reaction at the desired rate—a drop in absorbance per 15 s that is consistent throughout a 90 s trial. However, that intensity may not be appropriate for all thylakoid preparations at all concentrations so you will have to experiment with both the light intensity and the concentration of your thylakoid suspension to find a photosynthetic rate that does not use up all of our substrate (DCPIP) too quickly (before 90 sec.) or that is too slow to approach substrate depletion in 120 seconds. The 15 s reading intervals consists of 10 s of thylakoid illumination and 5 s to read the absorbance in the spectrophotometer. Your goal is to establish conditions favorable for a 90 s experiment. This data collection at interval of 15 s should yield 7 data points (i.e., absorbance readings at time = 0, 15, 30, 45, 60, 75, and 90 s) that are all in the linear portion of a curve when you graph absorbance (y axis) vs. time (x axis). If your light intensity is too high and/or your thylakoids too concentrated, your curve will flatten before the 90 second reading because you have depleted your DCPIP substrate and those data points after substrate depletion will be unusable. If your light intensity is too low and/or your thylakoids too dilute or of poor quality, the data points obtained within an optimal 90 sec. reaction will not measure enough of the linear part of the reaction. <br><br> | ||
Revision as of 17:54, 10 May 2012
Series 3- Lab 9 Photosynthesis -Photosynthetic Pigments and The Hill Reaction
In today's lab, you will measure the rate of electron transport in thylakoid membranes isolated from spinach chloroplasts using a procedure called the Hill Reaction. This procedure will allow you to measure the rate of oxygen evolution, and thus the rate of photosynthesis, in the thylakoids of isolated spinach chloroplasts.
Photosynthesis and Photosynthetic Pigments
Central to life on Earth is the photochemical process carried out by plants and cyanobacteria, wherein quanta of light are converted into chemical energy. In plants, light is absorbed by chlorophyll a and b and other antenna pigments of the light-harvesting complexes of Photosystems I and II.
Chlorophyll a and b impart the green color that one associates with plant leaves. Carotenoids, which are yellow pigments, are also present in leaves but are usually masked by the chlorophylls. It is only in the fall when the chlorophylls are degraded faster than the carotenoids that the yellow color becomes visible to us. The chlorophyll and carotenoid contents of plants can vary markedly with its age, or depend on environmental factors such as light intensity or quality during growth. The pale green appearance of a willow tree in early spring is markedly different from its olive-green of late summer. The intense dark green of "shade adapted" plants differs from the lacy green colors one sees at the top of a forest canopy.
Chlorophylls are found in the chloroplasts and are associated with the thylakoids, the internal membrane network of these organelles. It is now established that all chlorophylls are organized as discrete chlorophyll-protein complexes within the lipid matrix of the photosynthetic membrane.
The majority of chlorophyll a molecules (and all chlorophyll b and carotenoid molecules) function as antenna pigments. In combination with proteins, they form the light-harvesting complexes, which absorb and funnel light energy to the reaction center chlorophylls, thereby allowing the plant to utilize a broad spectrum of wavelengths for photosynthesis. Some of the chlorophyll a molecules serve specialized functions in the reaction centers of photosystems I and II, where the light energy is used to drive the reduction of components of the electron transport chain.
The energy from photons is passed via resonance energy transfer to a special pair of chlorophyll a molecules located in the reaction center, leading to the excitation and loss of electrons from these molecules. In each photosystem the excited electron from one reaction center chlorophyll is passed to the quinone primary electron acceptor thereby reducing it. The primary acceptor immediately donates its electrons to a neighboring molecule and so on through an electron transport chain to ultimately reduce NADP+ to NADPH. The resulting oxidized reaction center of photosystem II is able to split water molecules into protons, electrons and O2. The electrons extracted from water replace the electrons lost by the reaction center II chlorophyll. The reaction center chlorophyll of photosystem I is reduced by electrons coming from photosystem II.
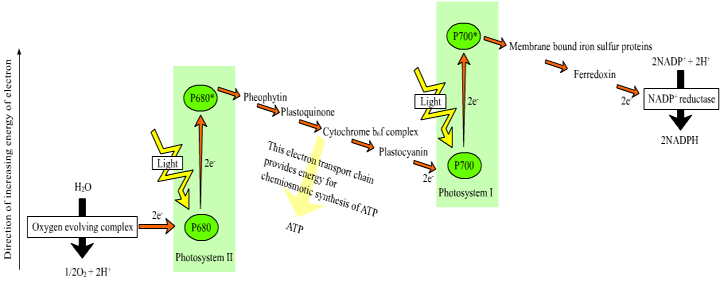
Figure 1. Process of electron transport in the light reactions of photosynthesis. Image from http://commons.wikimedia.org/wiki/File:Z-scheme.png
This electron transport is coupled in two steps to the formation of ATP through the mechanism of chemiosmosis. First, during the light reactions, the transport of electrons is coupled to the movement of protons from the stroma to the thylakoid lumen, forming a pH gradient across the thylakoid membrane. The sources of these protons are the splitting of water, which occurs on the lumenal side of the thylakoid membrane, and the transport of protons from the stromal side across the membrane into the lumen by the electron transport chain components plastoquinone and cytochrome b/f complex. In the second step, this gradient of protons is released when the protons diffuse through the membrane-spanning ATP-synthase molecule, which couples proton movement to the synthesis of ATP from ADP and Pi.
In today's lab, you will study the photosynthetic electron transport of thylakoid membranes isolated from spinach chloroplasts. During the isolation procedure, some of the more water-soluble components that function near the terminus of the main electron transport chain are lost. This makes it impossible to follow the production of ATP or the reduction of NADP+ in your preparations.
As is routine in studies using mitochondria and chloroplasts, we will supply an artificial electron acceptor to monitor "partial reactions" of the electron transport. These are chemicals that accept electrons at positions of the electron transport chain where exogenous compounds normally do not act in vivo. Frequently these electron acceptors are dyes specifically selected so their reduction can be monitored by spectrophotometry.
The dye reagent we are using in this experiment is 2,6-dichlorophenol indophenol (DCPIP). It is blue when oxidized and colorless when reduced. DCPIP accepts electrons between the electron chain components plastoquinone and cytochrome. The electrons are ultimately derived from water.
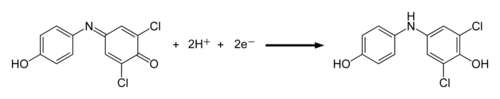
Figure 2. DCPIP reduction reaction
Figure 3. DCPIP acting as an electron acceptor. Animation from smabiology.blogspot.com/2008_12_01_archive.html
In 1937 Robert Hill showed that this partial reaction of the electron transport chain using DCPIP could be used to investigate the rate of oxygen evolution (from the splitting of water molecules in PSII) and thus the rate of photosynthesis in thylakoids of isolated chloroplasts. The reaction is now known as the Hill reaction and is still used today to determine photosynthetic rates in chloroplast preparations.
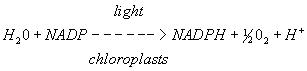
A. In vivo reaction
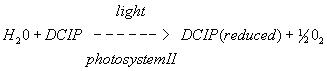
B. In vitro reaction
Figure 4. A. In vivo reduction reaction of NADP to NADPH in chloroplasts B. In vitro reduction reaction of DCPIP in photosystem II
Procedure
For the isolation of chloroplasts from spinach, a buffer is used since the leaves, when homogenized, can yield a low pH suspension. In the initial isolation step, the sorbitol serves to maintain an osmotic potential similar to that of an intact leaf. The divalent cation Mg2+, known to be important to membrane structure and function, is also included in the medium. All isolation and fractionation steps are performed at 4 degrees C to minimize proteolytic degradation of proteins.
In this lab you will examine the light reactions of photosynthesis by measuring the so-called Hill reaction in lysed chloroplasts. You will practice these measurements until you get reproducible results. Next week you will investigate the effect of various environmental factors on the light reactions of photosynthesis.
Be sure to keep all beakers and buffers on ice. Note that there are four different media used to prepare a thylakoid suspension: grinding medium (100 mM Tricine NaOH pH 7.8, 400 mM sorbitol, 5 mM MgCl2), breaking medium (20 mM Tricine NaOH pH 7.8, 5 mM MgCl2), resuspension medium (50 mM , 100 mM sorbitol, 5 mM MgCl2)and reaction solution (100 mM sorbitol, 5 mM MgCl2, 50 mM NaPO4, 0.05 mM DCPIP). Note the differences among them. Be sure to use the correct solution. Read labels carefully throughout the experiment.
Preparation of thylakoid stock solution
- Work in groups of eight students in steps 1 and 2. Throughout the procedure, be sure to wash glassware with water and the round bottom centrifuge tubes that have come in contact with chlorophyll using 70% ethanol. Please do this as soon as you have finished using this equipment.
- Prior to lab, 60 g of spinach leaves were mechanically macerated our lab specialists with 160 mL of grinding medium using Waring blenders. The ground up spinach will be given to you in a 500ml iced cold beaker. A group of 8 students will filter the spinach through first 2 layers of cheese cloth into another iced cold 500 ml beaker. You must gently squeeze the spinach in the cheese cloth to express the liquid and leave the pulp and debris in the cheesecloth. Discard the cheesecloth with the pulp in the trash can. Filter the expressed liquid (containing your intact cholorophasts) through 8 layers of cheesecloth into another 500ml cold beaker. Gently squeeze the cheesecloth to express the refiltered liquid, then discard the cheesecloth and pulp in the trash can. Clean Up: Rinse beakers with water immediately BUT DO NOT DISCARD THE FILTERED EXTRACT to be used in the next step!
- To isolate chloroplasts, divide the filtered extract into 4 equal portions and pour them into four 50 mL plastic round-bottom, capless centrifuge tubes. Balance each pair of tubes by transferring extract from the heavier tube to the lighter one, and centrifuge at 1000 x G in the SS34 rotor (see the conversion chart next to the centrifuge to convert RPMs to RCF which is also called g) for 5min in a Sorval refrigerated (4ºC) centrifuge. After the spin, each pair of students continues with one tube containing a pelleted chloroplast-rich fraction. From now on you are working in pairs. Carefully decant (pour off) the pale green supernatant and discard it. Save the green pellet (chloroplast-enriched fraction).
- Using a glass rod, gently resuspend the pellet by mixing 1–2 mL of breaking medium into the pellet. Make sure the pellet is completely detached from the wall and resuspended COMPLETELY. Avoid air bubbles (O2) that may oxidize enzymes and thereby reduce activity. The breaking medium is intended to shock the chloroplasts osmotically, thereby breaking open the organelles' outer membranes and releasing the stroma while leaving the thylakoid membranes intact. (Breaking medium: 20 mM Tricine NaOH pH 7.8, 5 mM MgCl2. Note the absence of sorbitol.)
- Bring the resuspended pellet to a volume of about 25 mL with cold breaking medium (50mL centrifuge tube will be about half-full), balance against a tube of water or another group, and centrifuge at 1900 x G for 5min. Discard the supernatant.
- Resuspend the resulting pellet containing your thylakoid-rich fraction in 1.5 mL of resuspension medium. This suspension is your stock preparation of thylakoids to be used in the Hill reaction. Keep it on ice. (Resuspension medium: 50 mM Tricine NaOH pH 7.8, 100 mM sorbitol, 5 mM MgCl2).
Table 1. Composition of Media
| Type of Medium | Tricine NaOH pH 7.8 |
Sorbitol | MgCl2 | Sodium Phosphate pH 6.8 |
DCPIP | |
|---|---|---|---|---|---|---|
| Grinding | 100mM | 400mM | 5mM | — | — | |
| Breaking | 20mM | — | 5mM | — | — | |
| Resuspension | 50mM | 100mM | 5mM | — | — | |
| Reaction | — | 100mM | 5mM | 50mM | 0.05mM |
Running the Hill reaction (work in pairs)
Materials: Each pair will have a ringstand with a test tube holder, and a 150W quartz-halogen projection lamp. You will have to find the best arrangement to measure the reduction of DCPIP (loss of blue color as decrease in absorbance at wavelength 580nm) accurately and reproducibly. A light meter and timer will be provided. Set up 5 ‘reaction tubes’ to begin with, adding 5 mL of reaction mixture, but do not add the thylakoid membranes, which must be kept on ice. Add these thylakoid membranes just before each reaction mixture is tested. [Reaction mix: 50 mM sodium phosphate, pH 6.8 buffer, 100 mM sorbitol, 5 mM MgCl2, 0.05 mM DCPIP]
Blanking the Spectrophotometer (Instructions are also found in Appendix B): Use a tube containing 50µL of your thylakoid suspension added to 5 mL of resuspension solution (NOT reaction solution!) to blank the Spec20 at 580 nm (filter level to the left). With Parafilm® over the top of the tube, invert once to mix, then wipe the tube with a Kimwipe® before blanking the instrument. Do not vortex. You may need to prepare a fresh blank periodically.
- Make sure your instrument has been warming up for at least 30min before use. If not turn it on with On-Off Knob (A) and wait.
- Set the wavelength to 580nm using Wavelength Selector (D).
- Insure that the filter lever located at bottom front of instrument is in correct position (to the left for wavelengths 340-599nm).
- Use Zero-Adjust Knob (A) to set meter to 0%T.
- Be sure that your blank (thylakoids in resuspension solution) is loaded in a 13mm test tube, not a plastic cuvette.
- Wipe outside of test tube with a Kimwipe, insert it into the Tube Holder (C), and close the lid.
- Use 100% Adjust Knob (B) to set meter to 100%T, which is equivalent to zero absorbance on the absorbance scale. Remove the blank tube and save it in your ice bucket.
Varying the Reaction Rate: The Hill reaction occurs at different rates depending on the light intensity and the quality and concentration of the thylakoids. Our goal today is to determine the best light intensity and thylakoid concentration for a 90 sec. reaction. You will measure the reaction rates by taking absorbance readings over that time period in 15 s intervals. We will use the drop in absorbance over time as a measure of reduction of blue DCPIP to colorless DCPIPH. If we can control all the variables properly, this reduction rate should also be an indication of the rate of photosynthesis since the light and dark reactions are coupled.
Depending on the number, distribution (clumped or not) and condition of thylakoids in your reaction tube, you may obtain varying reaction rates with a given light intensity. Therefore, you will test your thylakoids neat (undiluted) and diluted 1/2 with resuspension solution. We measure the incident light using a light meter in units of µmol photons m-2 s-1.
Typically, the light intensity is set at ~80 µmol photons m-2 s-1 in order to drive the reaction at the desired rate—a drop in absorbance per 15 s that is consistent throughout a 90 s trial. However, that intensity may not be appropriate for all thylakoid preparations at all concentrations so you will have to experiment with both the light intensity and the concentration of your thylakoid suspension to find a photosynthetic rate that does not use up all of our substrate (DCPIP) too quickly (before 90 sec.) or that is too slow to approach substrate depletion in 120 seconds. The 15 s reading intervals consists of 10 s of thylakoid illumination and 5 s to read the absorbance in the spectrophotometer. Your goal is to establish conditions favorable for a 90 s experiment. This data collection at interval of 15 s should yield 7 data points (i.e., absorbance readings at time = 0, 15, 30, 45, 60, 75, and 90 s) that are all in the linear portion of a curve when you graph absorbance (y axis) vs. time (x axis). If your light intensity is too high and/or your thylakoids too concentrated, your curve will flatten before the 90 second reading because you have depleted your DCPIP substrate and those data points after substrate depletion will be unusable. If your light intensity is too low and/or your thylakoids too dilute or of poor quality, the data points obtained within an optimal 90 sec. reaction will not measure enough of the linear part of the reaction.
Measurement of Hill Reaction Rates: For each reaction tube, do the following in turn.
- Label a 13mm glass test tube 1/2. Using your P200, add 100 microliters (0.1ml) resuspension solution. Mix the stock thylakoid suspension gently by flicking bottom of tube. Using a 1ml pipet (NOT your P200!) ,gently draw up and add 100 microliters of thylakoids to the equal volume of resuspension solution. Mix gently and keep both the stock and 1/2 dilution tube of thylakoids on ice.
- When you have set your light intensity to an even 80 µmol photons m-2 s-1 illumination of a tube that is held steadily in the tube holder apparatus you are ready to start your first trial reaction.
- Immediately remove 50 µL of the undiluted stock thylakoid suspension and add it to a reaction tube containing 5 mL of reaction mixture, mix gently by inverting once and place tube in the Spec20 to obtain a "dark reading" with the spectrophotometer set at 580 nm. This is the time = zero seconds absorbance reading. Expose the thylakoids to the light and take care not to shield the tube from the light with your hand.
- Take absorbance readings every 15 s (10 s illumination + 5 s reading) for 90 s (7 readings). Record absorbance readings and times in your lab notebook. Repeat the experiment at a light intensity of 100 µmol photons m-2 s-1, using undiluted thylakoids. Repeat the experiment using the 1/2 dilution of thylakoids at each light intensity, but run the lower light intensity for 2 minutes rather than for 90 seconds.
Compare your different trials to the following general standards:
A typical absorbance reading at time = 0 s is 0.8. If the drop in absorbance per 15 s interval is 0.05, then at time = 90 s, the absorbance reading should be 0.5.
Which, if any, of your light intensities and thylakoid concentrations best approximated those "standards"?
Continue doing 90 s runs under the light and thylakoid concentration conditions you have determined are best, until you obtain a consistent rate of DCPIP reduction. Be sure to hold your test tube in the same position for each 10 s of light exposure during the 90 s trial. If increasing or decreasing the light intensity and maintaining consistent positioning of the test tube does not work, please consult your instructor.
A description of simple linear regression and directions can be found in Appendix E Microsoft Excel Instructions for Regression Plots .
Plotting the Data: Please plot each trial as a scatter plot with a trendline in Excel with Time (s) on the x-axis and Absorbance at 580 nm on the y-axis. The reduction of DCPIP should be linear until DCPIP becomes limiting which is indicated by a leveling off of the absorption near the end of a 90sec reaction. Since you are interested in the maximum rate of DCPIP reduction, you are only concerned with the straight-line portion of the curve. With time in seconds plotted on the x-axis, the slope of the line (m value in the regression equation for your line y=mx+b) will reflect the change in absorbance per second. Change over time is rate; therefore, our photosynthetic rate is the slope you have obtained under optimal conditions.
Decide which light intensity and thylakoid conc. give a full 90sec reaction in the linear portion of the curve. You will start with those conditions next week when you perform your self-designed experiment that tests a variable that may affect photosynthetic rate, but keep in mind that different thylakoids suspensions can vary so you may have to adjust conc. or light intensity.
Self-Designed Experiment On A Variable Affecting Electron Transport Rate In Photosynthesis
Your instructor will post to your lab Sakai site a list of reference articles on variables affecting the Hill Reaction.
In Lab 10, you and your partner(s) will isolate thylakoids from spinach using the same protocol as you used today. You will test the effects of one environmental parameter on the Hill reaction rates. Please sign up today with your group to test the effects of one of the following parameters: temperature, light intensity, wavelength, inhibitors or uncouplers. See Lab 10 for additional information. You will work on a protocol as a group of 4, and in Lab 10 you will work as a team to complete the experiment using the.
All partners should take part in composing the proposal, which should include:
- A hypothesis with rationale and a plan for controlling confounding variables and/or acquiring an appropriate baseline for comparison.
- A list of reagents needed with protocols of how you will make all solutions and dilutions used in your experiment
- Your protocol (with sufficient detail, i.e., how to set up your tubes, etc.)
Your instructor will either approve your proposal, or suggest improvements.
Laboratory Cleanup
- Wash glassware with water and centrifuge tubes using 70% ethanol.
- Discard reaction mixtures in sink and test tubes in glassware disposal box.
- Glass pipettes should be left to soak in the pipette canisters, tips down.
- Place micropipette tips in the small orange bags at the bench.
- Pasteur pipettes and cover slips go in the glass container (blue cardboard box).
- Remove the last test tube from the sample chamber, and turn off your Spec20.
- Quit out of all applications on your computer.
Assignment
- If you have not had your protocol approved by your instructor for your experiment exploring a variable that affects the photosynthetic rate, arrange a time for you and your lab partners to meet with your lab instructor to discuss your plan. Alternatively, your instructor may prefer email communication. You need to plan your experiment early enough to leave time for feedback and modification of the proposal. Because, the prep staff need sufficient time to provide the materials you require, your compliance with the time frame your instructor sets is required. Late submissions will be penalized in the grade you receive for the research report you will write.
- The scientific research report that you will write on your experiment in Lab 10 will be a complete paper. Because students find converting lab manual protocol descriptions to a Materials and Methods section difficult, your instructors will provide you with an appropriate Materials and Methods section for the spinach thylakoid preparation and the Hill Reaction protocol that you performed today. Compare the wiki directions to this instructor written Materials and Methods version found at ?????. To begin to learn how to convert protocols to M&M, you should also refer to the Methods section in some of the journal articles that you will use as references to model what should be included or left out in M&M. Remember that concentrations of key reagents should be expressed as final (effective concentration) at crucial points in the experiment. You and your partners should use these models to write your proposed protocol for your LAB10 experiment in M&M form and submit this version as a group homework assignment (submit one M&M per group of 4 students) at the beginning of LAB 10. It will be graded out of 5 points. If you or your group is having trouble with calculating effective concentration or with the format for M&M, go to the BISC Help Room (SC267 S-Th evenings 8-10 pm) or make an appointment with one of the Science Writing Tutors using the on-line scheduler at | http://wellesley.mywconline.com/. The Science Writing specialists also have drop in hours in Sage Lounge.