BioBuilding: Synthetic Biology for Teachers: Lab 1: Difference between revisions
No edit summary |
No edit summary |
||
| Line 20: | Line 20: | ||
==Needed Materials== | ==Needed Materials== | ||
===Teacher Provides=== | ===Teacher Provides=== | ||
* | *Sterile toothpicks (or innoculating loop and bunsen burner) | ||
*Sterile tubes for growing liquid cultures of cells | *Sterile tubes for growing liquid cultures of cells, (4) if testing all 4 strains | ||
*Cuvettes to measure absorbances if spectrophotometer is not fitted for glass tubes | *Cuvettes to measure absorbances if spectrophotometer is not fitted for glass tubes | ||
* | *125 ml erlenmeyer flasks + stir bars, (4) if testing all 4 strains | ||
*Stir plates | *Stir plates, (4) if testing all 4 strains | ||
*Pipetmen and tips (P1000, P200, P20) | *Pipetmen and tips (P1000, P200, P20) | ||
*Pipets ( | *Pipets (5 ml) and bulbs | ||
*Timers | *Timers | ||
*Sharpies | *Sharpies | ||
*Nitrile or Latex gloves | *Nitrile or Latex gloves | ||
* | *Roller wheel at room temp or 37° for growing overnight cultures of bacteria (can use platform shaker instead) | ||
*If available, vortex for mixing cells prior to additions | *If available, vortex for mixing cells prior to additions | ||
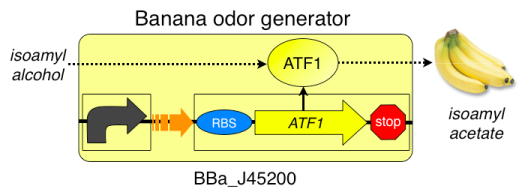
*If available, fume hood for measuring isoamyl alcohol (aka isopentyl alcohol) | *If available, fume hood for measuring isoamyl alcohol (aka isopentyl alcohol) | ||
===Kit Provides=== | ===Kit Provides=== | ||
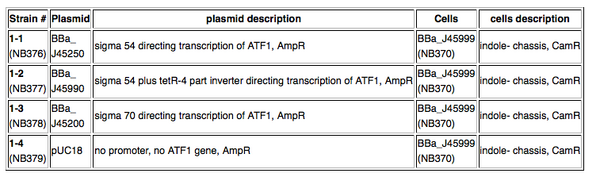
====4 strains (see table below)==== | ====4 strains (see table below)==== | ||
| Line 41: | Line 41: | ||
[[Image:Lab2 Kit.jpg|thumb|left|400px]] | [[Image:Lab2 Kit.jpg|thumb|left|400px]] | ||
=====Room Temperature===== | =====Room Temperature===== | ||
*Concentrated Banana Extract to make Banana smell standards | |||
*Concentrated Banana | |||
=====4° (fridge)===== | =====4° (fridge)===== | ||
* | *Ampicillin (100 mg). Can be added to 1 ml in H2O, filter sterilized and used at a 1:1000 dilution in LB. Alternatively, can add entire aliquot to 1 liter of LB and store LB + amp in the fridge. | ||
=====Chemical Hood===== | =====Chemical Hood===== | ||
* 700 ul isoamyl alcohol, enough to prepare 1L of media for lab | * 700 ul isoamyl alcohol, enough to prepare 1L of media for lab | ||
Revision as of 20:00, 9 January 2012
|
Eau That Smell Lab notes |
PDF of this page
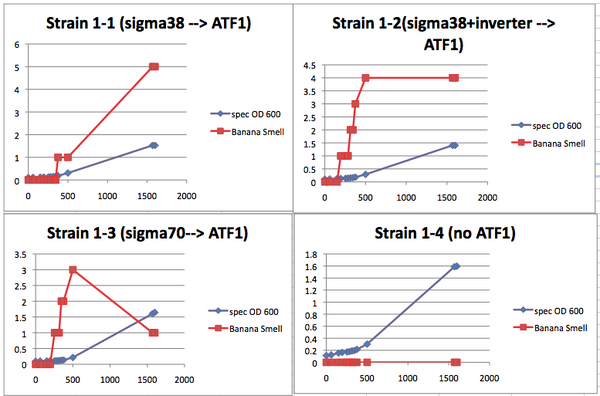
Note: This lab is also described in this Methods in Enzymology chapterTeacher ConsiderationsThis lab provides a valuable opportunity to teach microbiology techniques, population growth dynamics, molecular genetics and basic synthetic biology concepts in a meaningful, real world way. As can be seen in the discussion questions for the lab report, the analysis of the lab will provide the students with a chance to do meaningful error analysis and examine the difference between quantitative results and qualitative results. This lab offers two different protocols based on the time the teacher wishes to allow. Each of these protocols covers the same concepts but allows for different emphases. Protocol A--Condensed data collection: This is a shorter procedure for the students. This shorter protocol emphasizes data analysis over data collection. With this protocol the teacher can choose to have the students do the initial bacterial culturing if more microbial techniques are to be emphasized. Essentially, a day prior to any data collection, the large cultures are set up. Part of the starter culture is immediately removed and placed in the refrigerator. This serves as the lag phase sample. After 5-7 hours, a second sample is removed. This serves as the log phase sample. The last third of the culture should be allowed to grow overnight. This serves as the stationary phase sample. The samples can then be provided to the students the following class day, allowing the students to collect data in single lab period. Go to Protocol A for the detailed protocol. Protocol B--Growth curve data collection: This version provides a greater emphasis on collection of growth curve data. In this version, the students subculture from the overnight samples and then assess the banana smell and turbidity (population) of the subcultures every twenty minutes. To increase the number of data points, different classes can measure the same subcultures throughout the day. Alternatively, the subcultures can be refrigerated and warmed back to room temp for 30 minutes prior to the next data point collection. You should expect to collect data over three days to get data points from all phases of the growth curve. Go to Protocol B for the detailed protocol. Both procedures include instructions for using a spectrophotometer to measure the population growth. If a spectrophotometer is not available, the population can be easily measured using the McFarland Turbidity methodology. Instructions for this measurement are also included with each protocol. An introductory power point that can be adapted to either protocol can be found here. Also useful might be the MIT iGEM team presentation [video] and their ppt. Needed MaterialsTeacher Provides
Kit Provides4 strains (see table below)
Chemicals Room Temperature
4° (fridge)
Chemical Hood
AssessmentLab Report RubricLab Report ScoreSheetSurveyTo help us improve the labs, you can
Thanks! Variations to try
FeedbackWe're always looking to hear back from you if you've thought about this unit, tried it, or stumbled across it and want to know more. Please email us through BioBuilder, info AT biobuilder DOT org. Navigation
|