BioBuilding: Synthetic Biology for Teachers: Lab 1: Difference between revisions
No edit summary |
No edit summary |
||
| Line 5: | Line 5: | ||
[[Image:Copy of BBa J45200.png]] | [[Image:Copy of BBa J45200.png]] | ||
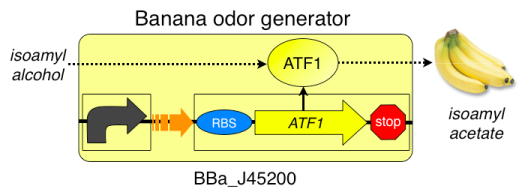
=====Note: This lab is also described in this [[Media:MiE 00012.pdf |Methods in Enzymology chapter]]===== | =====Note: This lab is also described in this [[Media:MiE 00012.pdf |Methods in Enzymology chapter]]===== | ||
==MIT iGEM team presentation== | |||
<embed id=VideoPlayback src=http://video.google.com/googleplayer.swf?docid=3938737372714460591&hl=en&fs=true style=width:400px;height:326px allowFullScreen=true allowScriptAccess=always type=application/x-shockwave-flash> </embed> | |||
<br> | |||
[[Media:BioBuilding IGEM2006-MIT-Powerpoint.ppt| ppt]] | |||
== Teacher Considerations == | == Teacher Considerations == | ||
This lab provides a valuable opportunity to teach microbiology techniques, population growth dynamics, molecular genetics and basic synthetic biology concepts in a meaningful, real world way. As can be seen in the discussion questions for the lab report, the analysis of the lab will provide the students with a chance to do meaningful error analysis and examine the difference between quantitative results and qualitative results. | This lab provides a valuable opportunity to teach microbiology techniques, population growth dynamics, molecular genetics and basic synthetic biology concepts in a meaningful, real world way. As can be seen in the discussion questions for the lab report, the analysis of the lab will provide the students with a chance to do meaningful error analysis and examine the difference between quantitative results and qualitative results. | ||
Revision as of 15:51, 24 July 2011
|
Eau That Smell Lab notes |
PDF of this page
Note: This lab is also described in this Methods in Enzymology chapterMIT iGEM team presentation<embed id=VideoPlayback src=http://video.google.com/googleplayer.swf?docid=3938737372714460591&hl=en&fs=true style=width:400px;height:326px allowFullScreen=true allowScriptAccess=always type=application/x-shockwave-flash> </embed>
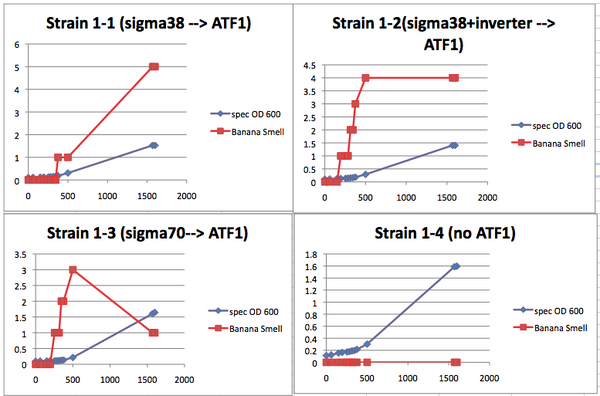
Teacher ConsiderationsThis lab provides a valuable opportunity to teach microbiology techniques, population growth dynamics, molecular genetics and basic synthetic biology concepts in a meaningful, real world way. As can be seen in the discussion questions for the lab report, the analysis of the lab will provide the students with a chance to do meaningful error analysis and examine the difference between quantitative results and qualitative results. This lab offers two different protocols based on the time the teacher wishes to allow. Each of these protocols covers the same concepts but allows for different emphases. Protocol A--Condensed data collection: This is a shorter procedure for the students. This shorter protocol emphasizes data analysis over data collection. With this protocol the teacher can choose to have the students do the initial bacterial culturing if more microbial techniques are to be emphasized. Essentially, a day prior to any data collection, the large cultures are set up. Part of the starter culture is immediately removed and placed in the refrigerator. This serves as the lag phase sample. After 5-7 hours, a second sample is removed. This serves as the log phase sample. The last third of the culture should be allowed to grow overnight. This serves as the stationary phase sample. The samples can then be provided to the students the following class day, allowing the students to collect data in single lab period. Go to Protocol A for the detailed protocol. Protocol B--Growth curve data collection: This version provides a greater emphasis on collection of growth curve data. In this version, the students subculture from the overnight samples and then assess the banana smell and turbidity (population) of the subcultures every twenty minutes. To increase the number of data points, different classes can measure the same subcultures throughout the day. Alternatively, the subcultures can be refrigerated and warmed back to room temp for 30 minutes prior to the next data point collection. You should expect to collect data over three days to get data points from all phases of the growth curve. Go to Protocol B for the detailed protocol. Both procedures include instructions for using a spectrophotometer to measure the population growth. If a spectrophotometer is not available, the population can be easily measured using the McFarland Turbidity methodology. Instructions for this measurement are also included with each protocol. Needed MaterialsTeacher Provides
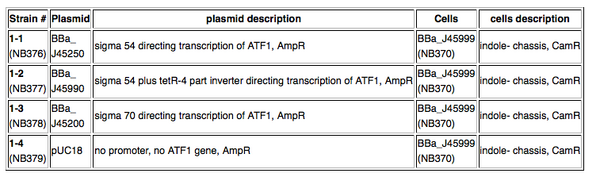
Kit Provides4 strains (see table below)
Chemicals Room Temperature
4° (fridge)
Chemical Hood
AssessmentLab Report RubricLab Report ScoreSheetSurveyTo help us improve the labs, you can
Thanks! Variations to try
FeedbackWe're always looking to hear back from you if you've thought about this unit, tried it, or stumbled across it and want to know more. Please email us through BioBuilder, info AT biobuilder DOT org. Navigation
|