EGTA: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
storage |
illustration |
||
| Line 1: | Line 1: | ||
[[Image:EGTA.png|thumb|right|300px|chemical structure of EDTA - ethylene-glycol-tetraacetic acid]] | |||
'''Ethylene glycol tetraacetic acid (EGTA)''' is a common buffer ingredient due to its chelating activity. It is similar to the better known [[EDTA]], but has a much higher affinity for calcium ions than for magnesium ions. Buffers made with EGTA are used in some cases to mimic the environment inside living cells where calcium ions are usually at least a thousandfold less concentrated than magnesium ions. | '''Ethylene glycol tetraacetic acid (EGTA)''' is a common buffer ingredient due to its chelating activity. It is similar to the better known [[EDTA]], but has a much higher affinity for calcium ions than for magnesium ions. Buffers made with EGTA are used in some cases to mimic the environment inside living cells where calcium ions are usually at least a thousandfold less concentrated than magnesium ions. | ||
Revision as of 15:26, 15 June 2009
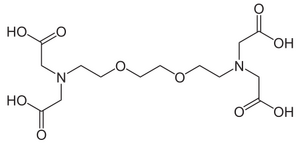
Ethylene glycol tetraacetic acid (EGTA) is a common buffer ingredient due to its chelating activity. It is similar to the better known EDTA, but has a much higher affinity for calcium ions than for magnesium ions. Buffers made with EGTA are used in some cases to mimic the environment inside living cells where calcium ions are usually at least a thousandfold less concentrated than magnesium ions.
Full chemical name: ethylene glycol-bis(2-aminoethylether)-N,N,N',N'-tetraacetic acid
EGTA 0.5M stock solution
100 ml solution
- 19g EGTA (MW 380g/mol)
- ddH2O to 90ml
- adjust pH 7.5/8.0
- adjust volume to 100ml
Storage
- room temperature
- for years
See also
- Wikipedia: EGTA
- Sigma catalogue EGTA E4378 100g 220€, E3889 100g 253€ (as of 6/'09)