Shreffler:BioCSI Basophil Activation
From OpenWetWare
Jump to navigationJump to search
Overview
Basophil activation in unfractionated samples such as PBMC (note basophils are less dense the Ficoll) or whole blood can be measured by changes in the expression of cell surface markers or even intracellular events (e.g. phosphorylation, oxidative burst, calcium flux) by flow cytometry. Separate markers (e.g. as CD123+ HLA-DR-) are used to specifically identify basophils in addition to those used for assessment of activation.
This protocol is specifically for the BioCSI study.
Materials
- RPMI medium (cellgro, 10-040-CV; store at 4°C in dark)
- 1 X FACS lysing solution (made from 10X stock with dH2O; store at 4°C; expires in 1 week)
- PBS + 20 mM EDTA (sterile filter, store at 4°C; expires in 1 week)
- Staining Buffer (PBS + 2 mM EDTA + 0.5% BSA) (sterile filter, store at 4°C; aliquot in hood; expires in 2 months)
- desired monoclonal antibodies (e.g. CD63-FITC, CD203c-PE, CD123 PE-Cy5, CD69-APC-Cy7, HLA DR-PE-Cy7, CD3-APC, CD14-APC, CD19-APC, CD41a-APC)
- stimulant aliquots (pre-made, 30 μL aliquots, distributed by Mt. Sinai; stored at -20°C)
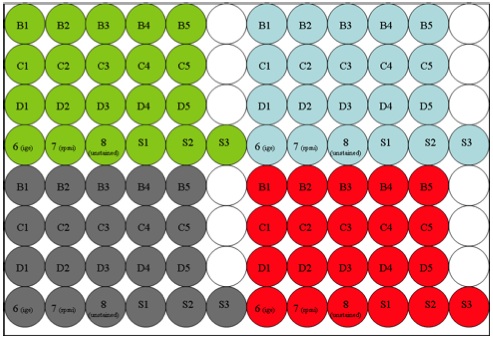
(The green, blue, grey, and red colors represent 4 separate samples. Set up the plate for the number of samples received at one time.)
Procedure
- Obtain whole blood specimens collected in sodium heparin tube (green top).
- Record the total volume of blood in the wiki.
- Thaw 1 EW and 4 CR (30 ul) antigen aliquots. Label them A1-A5 (Ova=A1 and the CR aliquots=A2-A5).
- Add 30 μL of RPMI to A1-A5 aliquots.
- Set up a 96-well plate for serum and antigen dilutions.
- Prepare serum/antigen dilutions as follows.
- Add 100 μL RPMI to S1, S2, and S3.
- Make serum dilution #1 by adding 100 μL serum to S1.
- Make successive 3 fold dilutions by transferring 50 μL from S1 to S2. Mix. Transfer 50 μL from S2 to S3 and mix.
- Add 60 μL of S1 to A1 and A2. Vortex.
- Add 60 μL of S2 to A3. Vortex.
- Add 60 μL of S3 to A4. Vortex.
- Add 60 μL RPMI to A5. Vortex.
- Incubate Eppendorf tubes A1-A5 and plate for 30 min at 37°C
- Add 270 μL RPMI to wells B1-D5.
- Add 300 μL RPMI to wells 6-8.
- Add 0.6 μL anti-IgE to well 6 (2 μg/mL).
- Incubate 2.5 mL of RPMI to be used for diluting tubes A1-A5 after 30-minute incubation.
- While samples are incubating…
- Spin whole blood from the green tip heparin tube at 300 x g for 10 min. Take off supernatant and fill with PBS to wash. Invert.
- Spin the sample again for 10 min at 300 x g. Take off supernatant.
- After antigen/serum incubation…
- Add 480 μL RPMI to A1-A5 and vortex (use the 2.5 warm RPMI from step 11).
- Transfer 30 μL from “A” tubes to corresponding “B” tubes (A1 to B1, A2 to B2, etc.) with 50p multi-channel pipette. Mix.
- Transfer 30 μL from “B” tubes to corresponding “C” tubes and mix.
- Transfer 30 μL from “C” tubes to corresponding “D” tubes and mix.
- These dilutions of Ag:Serum are now used 1:1 with washed cells in usual basophil activation protocol
Discussion
References
- Hennersdorf F, Florian S, Jakob A, Baumgärtner K, Sonneck K, Nordheim A, Biedermann T, Valent P, and Bühring HJ. Identification of CD13, CD107a, and CD164 as novel basophil-activation markers and dissection of two response patterns in time kinetics of IgE-dependent upregulation. Cell Res. 2005 May;15(5):325-35. DOI:10.1038/sj.cr.7290301 |
- Knol EF, Mul FP, Jansen H, Calafat J, and Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991 Sep;88(3 Pt 1):328-38. DOI:10.1016/0091-6749(91)90094-5 |
- Shreffler WG. Evaluation of basophil activation in food allergy: present and future applications. Curr Opin Allergy Clin Immunol. 2006 Jun;6(3):226-33. DOI:10.1097/01.all.0000225165.83144.2f |
Contact
- Who has experience with this protocol?
- Email Wayne Shreffler through OpenWetWare