Sean Lauber:ELISA Development
First you need to determine what reagents you will use for your ELISA. The protocol I use is based off of a generic R&D ELISA protocol. The primary antibody binds to the plate (this should be monoclonal) (note that you'll need specialized plates that are treated and can bind to antibodies), the sample binds to the primary, then the secondary binds to the sample (this should be be biotinylated), then strepavidin-HRP binds to biotin and the HRP is used for color development (see any R&D ELISA for the complete protocol).
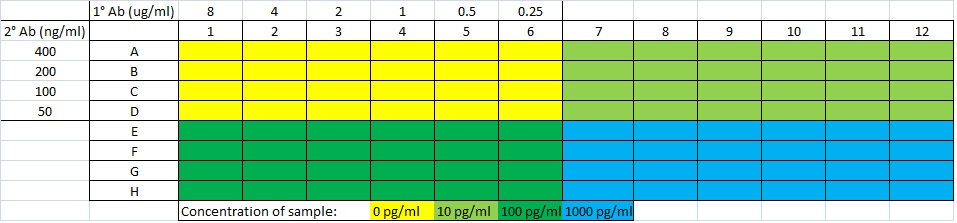
Next you need to determine what concentration of primary and secondary will work best for giving good color development for a range of sample concentrations. In the past, I have chosen the range 0.25 - 8 μg/mL for the primary, 50 - 400 ng/ml for the secondary, and 0, 10, 100, 1000 pg/ml for sample detection. Set up the plate as follows (checkerboard ELISA):
Then you want to determine what concentrations of primary/secondary give the highest change between standard concentrations (so 10 - 0, then 100 - 10, then 1000 - 100 pg/ml), this is the concentration you want to use. Usually the highest concentration of both primary/secondary works best but if reagents are expensive you might want to reconsider.
At this point it's a good idea to repeat the optimal concentration of primary/secondary with what you might use for a standard curve (so instead of 0, 10, 100, 1000, try 0, 5, 10, 20, 40, 80, 160, 320, 640 or something), do these samples in duplicate/triplicate/quadruplicate (the more the better). You want to make sure that the standard curve you get out of this is linear and you can identify the limit of detection. Most ELISAs I've seen aren't optimized for detection under about 7 pg/ml, so if you're able to detect around this range, you're doing good. I would then try repeating this again in order to make sure you're able to get reproducibility. If your standard is not detectable at 10 pg/ml then you may not be able to detect this low. You can play around with things like buffers and washes and incubation times and %FBS but that's beyond the scope of this wiki.
Also a good idea at this point to do a test for specificity. Test varying concentrations of different proteins that show some similarity or proteins you suspect may cross-react with the antibody. Show that the antibody pair you're using is specific. Use a high concentration (10 ng/ml) to really show you're not picking it up.