Protoplast Production
-
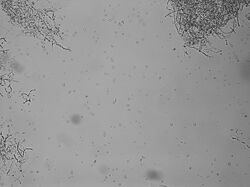
Protoplasts being released from hyphae.
-
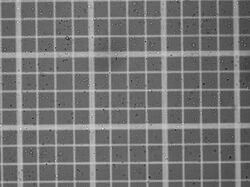
Protoplasts on a hemocytometer.
-
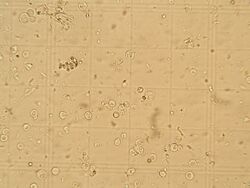
Protoplasts on a hemocytometer.
- Inoculate 50 mL PDY or MPDY in a 125 mL flask with 9 plugs of leading edge tissue. Culture at 30C, 70 rpm for 5 days.
- Prepare 20ml of lysing enzymes (0.04g/ml of Lysing Enzyme from Trichoderma harzanium in 0.6 M KCl, 0.1 M phosphate buffer pH 5.8) and incubate 15min at 37C, 200 rpm (use a 50 ml tube)
- Collect 5 day old mycelium from the flask using a 40um filter and wash it using 10ml of 0.6 M KCl, 0.1 M phosphate buffer pH 5.8 (use a 50 ml tube and 40 um filter).
- A second wash can sometimes improve yield with older cultures: resuspend the mycelia in 10ml of 0.6 M KCl, 0.1 M phosphate buffer pH 5.8 in a 50 mL conical tube, vortex, and then centrifuge at 6k rpm for 5 minutes. Discard the supernatant.
- Transfer the mycelium into a single 125 ml flask,
- Filter lysing enzymes (0.2um syringe filter) and add the enzyme to the flask with mycelia. Incubate for 3-4h, 25C, 70 rpm.
- Collect 10 µL and check for protoplast formation.
- Filter the protoplasts through sterile cheese cloth stuffed into a 60 mL syringe that is directed into a 40um cell filter over a 50 mL conical tube.
- If needed filter through a 5 um MILLEX syringe filter into a clean conical tube.
- Collect the protoplasts by centrifugation, 3500 rpm, 4C
- Discard the supernatant and wash the protoplasts using 10ml of TMS
- Collect the protoplasts by centrifugation, 3500 rpm, 4C
- Discard the supernatant and resuspend the protoplast into 300ul of TMSC
- Count the protoplasts
- Using microscope at 40x and Neubauer improved 0.1mm 0.0025 mm^2
- Image all four large corner squares each composed of 16 small squares.
- Using Fiji count protoplasts in all 64 squares.
- http://insilico.ehu.eus/counting_chamber/neubauer_improved.php
- https://www.emsdiasum.com/microscopy/technical/datasheet/68052-14.aspx
- Adjust the protoplast accordingly using TMSC.
Reagents list and recipe:
Autoclave Store at 4 ° C in 50 ml falcons.
0.6 M KCl / 0.1 M NaP 500 ml 0.6 M KCl + 50 ml 1 M NaP pH 5.8. Autoclaved (1 +2) (1) 0.6 M KCl: 22.37g KCl in 500 ml of H2O. Autoclaved. (2) 1M NaP pH 5.8: 15.8ml 1M Na2HPO4 + 184.2ml 1M NaH2PO4 pH 5.8 (NaOH pellets at the start). Autoclaved.
(3 + 4) (3) 1 M Na2HPO4: in 50 ml H2O. Autoclaved. (4) 1 M NaH2PO4: in 500ml of H2O. Autoclaved.
TMS Sorbitol 1 M MOPS 10 mM 18.2g of Sorbitol + 209 mg MOPS per 100 ml of H2O, pH 6.3 (NaOH). Sterilize by filtration.
TMSC Sorbitol 1 M MOPS 10 mM CaCL2 40 mM 18.2g of Sorbitol + 209 mg MOPS + 588 mg CaCl2 per 100 ml of H2O, pH 6.3 (NaOH). Sterilize by filtration.
MS Sorbitol 0.6 M MOPS 10 mM 10.9g of Sorbitol + 209 mg MOPS per 100 ml of H2O, pH 6.3 (NaOH). Sterilize by filtration.
TE CaCl2 2X, pH 7.5 2 M Tris pH 8 1 ml 0.5 M EDTA 0.4 ml CaCl2, 2H2O 80 mM 1176 mg H2O qs 100 ml. Adjust the pH to 7.5 (HCl). Filter. Aliquot by 10 ml.
PEG 60% Prepare immediately. 1.2 g of PEG6000 + 800 µl of MS (= 2 ml of final volume) in 14ml Falcon tube. Dissolve in the microwave for a few seconds. Let cool to room temperature.