Protocols isolate cancer microparticles
Overview
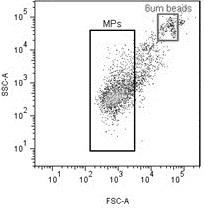
Microparticles or microvesicles are small blebs of membranes (typically defined as 0.1 to 1 or even 1.5 um in diameter, although this definition is arbitrary and under debate) shed from numerous cancer cell types. The biological functions of these are under investigation 1.
Materials
- Typical cell culture lab equipment: sterile hood, 37°C incubator with 5% CO2, petri dishes, pipettes, centrifuge tubes etc.
- Cancer cell line--numerous types are available
- Media incubating cancer cell line supplemented with FBS (aka FCS; optimal % FBS varies by cell type) and optional antibiotics (i.e. Penicillin/Streptomycin)
Equipment
- Centrifuge capable of 1500g (optional cooling to 10°C)
- Ultracentrifuge if desiring to wash
Procedure
- Change media when cancer cells are approaching 75% confluence.
- Collect the incubation media after 24 hours* into a centrifuge tube.
- Centrifuge at 500-1500g for 10 minutes.
- Collect the from the top 3/4th to 4/5th of the media, discarding the pellet of loose cells/large debris. DO NOT simply dump from the top, unless you don't mind having to repeat step 3.
- Centrifuge again at 1500g for 15 minutes, collect from the top, discarding the pellet
- Ultracentrifuge at 20000g for 10 minutes to pull down large MPs. A longer/harder spin is required for pulling down smaller MPs, but for flow cytometry, this is enough to isolate MPs smaller than most flow cytometers can measure3.
- Wash and resuspend pellet in PBS or whatever else you want.**
- Snap freeze over liquid nitrogen and store in -80°C for long-term (weeks to months), -20°C for shorter term (days)
Notes
Happy to have your positive input!
'* The cells can be at a lower confluence and the incubation time can be longer. For example, I was able to get enough MPs for flow cytometry from 50% confluent cells after collecting the media that had been incubating the cells for 48 hours.
'** Ultracentrifugation does something to the 'stickiness' of MPs. For example, centrifugation at 10000g at 10°C for 30 minutes is not able to create a visible pellet from MDA MB-231 cancer MP media obtained by this protocol. However centrifugation at 30000g at 10°C for 60 minutes of the same media created a visible pellet, after which resuspended, a 10000g spin at room temperature for 4 minutes resulted in a visible pellet.
References
1. Semin Thromb Hemost. 2010 Nov;36(8):888-906. Microparticles in cancer. Rak J. PMID 21049390
2. Platelets. 2008 Aug;19(5):365-72. Flow cytometric measurement of microparticles: pitfalls and protocol modifications. Shah MD, Bergeron AL, Dong JF, López JA. PMID 18791943
3. Transfus Med Rev. 2006 Jan;20(1):1-26. Cell membrane microparticles in blood and blood products: potentially pathogenic agents and diagnostic markers. Simak J, Gelderman MP. PMID 16373184
Contact
- Who has experience with this protocol?
or instead, discuss this protocol.