Physics307L F09:People/Ierides/The Balmer Series
SJK 00:53, 30 November 2009 (EST)

Overall, there are a lot of good parts here, and a strong foundation, including good data, analysis, and some good starting figures. However, there's a lot of work to be done. The most serious issue is noted below in the abstract / introduction, regarding wording. That may be easily fixed, but absolutely must be. Next most important, and probably more difficult is the results and discussion. It needs to be overhauled, with many new figures / tables. I think reading some peer-reviewed physics or astronomy papers of interest to you would help you get an idea of what's required. For your "extra data" session, I'm suggestion pursuing the Hydrogen versus Deuterium issue (see below), but you could choose to do other things of interest as well.
The Balmer Series of Hydrogen and Deuterium in Atomic Physics
SJK 00:49, 30 November 2009 (EST)

Good title, author, contact.
Author: Anastasia A. Ierides
Experimentalists: Anastasia A. Ierides, Alexandra S. Andrego
Institution: University of New Mexico
Address: University of New Mexico, Department of Physics and Astronomy, Albuquerque NM, 87106
Abstract
SJK 22:31, 29 November 2009 (EST)

This abstract relies way too much on other sources, and some sentences are far too identical to wikipedia to use them without quotations and citing the source. E.g., "in turn led to the finding of the Lyman, Paschen, and Brackett series used in predicting the absorption/emission lines of hydrogen found outside the visible spectrum." In science, that would be looked at very negatively. I think you need to write the abstract starting over from scratch without looking at those sources. We'll talk about the abstract some more in class tomorrow, I think.
Before 1885, the year that the Balmer formula was founded by a Swiss school teacher Johann Jakob Balmer, physicists, although aware of atomic emissions, lacked the tools to predict the location of each spectral line. The Balmer equation is used in the prediction of each of the four visible emission/absorption lines of hydrogen with high precision. This had inspired the Rydberg equation, invented by a Swedish physicist Johannes Robert Rydberg. This new equation was a generalization of the Balmer formula, which in turn led to the finding of the Lyman, Paschen, and Brackett series used in predicting the absorption/emission lines of hydrogen found outside the visible spectrum.
According to the Rutherford Bohr model (devised by Neils Bohr in 1913 from the amelioration of a model created by Ernest Rutherford in 1911) of the Hydrogen atom, an electron transition that occurs between the second energy level or first excited state in the atom (corresponding to n=2) and any other higher energy level results in the Balmer lines.
The Balmer series has been helpful in astronomical and physical use for years due to the abundance of hydrogen in the universe. It has been used for several means such as spectral classification, the measure radial velocities of objects in space due to doppler shifting, and the distances to those objects.
During our experiment we found that slight discrepancy in our values could be due to some oversight when using the 'scope'. There might have gear back lash from not turning the knob all the way back before remeasuring spectra for each trial, even though we took great care in doing so. Also, the lab lasted over two days, with a week interval and during that interval another group had used the same device used for our experiment, so re-calibration for our set of data was necessary during the second day.
Introduction
SJK 22:41, 29 November 2009 (EST)

Compared with the abstract, this seems more like your original writing (though it still would be a good idea to write from scratch with less fresh wikipedia in your head. Also, I'd note that much of your abstract actually belongs here in the introduction. Your introduction will end up longer and will have more citations to peer-reviewed publications (wikipedia does not count).
The Balmer series is one of six series in which the spectral line emissions of hydrogen are designated. There are four different emission wavelengths of visible light by which the hydrogen spectrum is defined. These wavelengths can be calculated using the Balmer formula (found by Johann Balmer, 1885) written above in the "Purpose" and reflect emissions of photons by transitions of electrons between principal quantum number levels from [math]\displaystyle{ n\geq 3 }[/math] to [math]\displaystyle{ n=2\,\! }[/math]. [1]
Compared to the Hydrogen atom, which contains one proton in the nucleus, the Deuterium atom, contains a proton and a neutron in its nucleus. Thus the Deuterium atom is heavier than the regular Hydrogen atom. SJK 22:43, 29 November 2009 (EST)

This is sort of an orphan thought. Including information about H versus D in your introduction will be very appropriate. However, you will need to go further and state why it matters (different reduced mass shifts spectra lines).
By observing and classifying spectra lines of the hydrogen and deuterium atoms the Balmer series can be determined. By using electrical stimulation to excite the atoms to higher energy levels we can measure the emitted photons of wavelengths equivalent to the energy of our excited electrons. Through this lab and our measurements we were able to experimentally determine Rydberg's constant, R, that is used in the Hydrogen Spectrum equation:
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}) }[/math]
- [math]\displaystyle{ n=3,4,5,...\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}) }[/math]
Or more generally a modified version of the above equation:
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{m^2}-\frac{1}{n^2}) }[/math]
- [math]\displaystyle{ m=1,2,3,...\,\! }[/math]
- [math]\displaystyle{ n=2,3,4,5,...\,\! }[/math]
- [math]\displaystyle{ n\gt m\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{m^2}-\frac{1}{n^2}) }[/math]
This process is applied to both hydrogen vapor and deuterium vapor.
SJK 22:44, 29 November 2009 (EST)

It ends abruptly, and both of these formulas are not important, I don't think.
Materials and Methods
SJK 00:31, 30 November 2009 (EST)

The style of your materials and methods is not in-line with typical formal scientific report. In those, you typically do not list materials separate from procedures, but instead write them together. So, what you'll want to do is to break this section up into different tasks (e.g. Spectrometer Alignment and Calibration; Data Collection; Data Analysis). Then, within each of those sections write the methods as prose that includes direct mention of the instruments and materials used. For example, in the data analysis section, you'd write something like, "The mean and standard error of the mean for multiple measurements of the same spectral line were calculated using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA)." Or in another section, "All spectral sources were power by a Spectral Tube Power Supply (Model SP200, [company], [location]). For any given measurement, the bulbs were allowed to warm up for at least __ minutes...etc."
This is also a good time to point out that you should describe your data analysis methods in the "Materials and Methods" section, which I think as of now you don't. I also think you probably don't have enough detail in the sections you do have here now.
Constant-Deviation Spectrometer (SER. #12610):
- Spectrum Tube Power Supply (Model SP200)
- 5000V
- 10 MA
Underlined Spectrum Tubes:
- Spectrum Tube Power Supply Model SP200 5000V
- Spectrum Tube, Mercury Vapor S-68755-30-K
- Spectrum Tube, Hydrogen S-68755-30-G
- Spectrum Tube, Deuterium S-68755-30-E
For this lab we are using Gold's Lab Manual Gold's Physics 307L Manual
SJK 00:24, 30 November 2009 (EST)

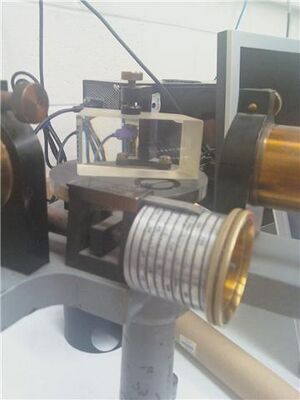
These figures are very helpful for understanding how you did the experiment. In a traditional publication, these kinds of photos are usually omitted (probably due to space/page costs). However, I think it'd be good for you to keep them. To make them better, you need to: (a) number every figure, and refer to the figure by number in text. For example, "...the slit width was adjusted blah blah (slit shown in Figure __)." and (b) Figure captions need more detail. You don't need to explain everything, but you need enough information that the reader can understand what the photo is and why you are showing it. For example, "Figure ___: Working experimental setup. The source ___ (glowing vertically on the left) emits light through slit. Light is dispersed and reflected by the pellin-broca pism (center), and focused through eyepiece (front)." Actually editing the photos to put labels directly on the photos would help.
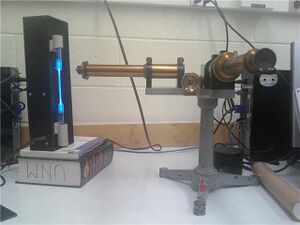

- First we adjusted the spectrometer, bringing the cross-hairs into focus by sliding the ocular to suit our vision
- Then we brought the slit into focus, turning the large ring near the center of the viewing telescope making sure to turn the screw in only one direction to avoid error due to "gear back lash"
- Next, we attached the mercury bulb and turned on the mercury tube power supply to let it warm up
- Using the spectrometer with a wide slit, we found a line of the mercury spectrum and then narrowed the slit until the line became narrow and sharp
- Then we located as many mercury spectra as we could and noted the orientation and value of our spectrometer dial
- While using the wavelengths of light given in Gold's Manual (page 29), we finished calibrating the system
- To solve for Rydberg's constant we correlated our data to the appropriate quantum numbers and used the equation given (page 30)
- Finally, we repeated this process for deuterium as well.
- Note: The first two steps are to make certain that no parallax exists between the cross-hairs and the slit when in sharp focus
Table taken from page 29 of this link [2]
Color Wavelength (nm) Deep Violet (very hard to see) 404.7 Violet 435.8 Very Weak Blue-Green skip Green 546.1 Yellow 1 577.0 Yellow 2 579.0 Red 690.75
SJK 00:35, 30 November 2009 (EST)

This should be where your results begin. Raw data are results. In your case, I think they're results worthy of displaying. What you should do is divide the following table up into multiple tables and figures (graphs). You want each table or graph to represent something important in a way that the reader can very easily follow. If you want to include this entire spreadsheet as well, you can link that as an appendix or footnote link to another document.
This is our raw data:
Using our raw data tables we used the functions in Excel for mean and standard deviation to find the standard error margins. From that we formulated a total mean:
Results and Discussion
SJK 00:39, 30 November 2009 (EST)

Notice that besides the photos of the apparatus above, you do not have any other figures. Whereas in almost any experimental research report, there will be several figures to cleanly and clearly display the results. So, definitely you're going to need to create graphs from the data, as I mentioned above. Also, this results section right now looks like a string of calculations, which would be fairly confusing to the reader. Plus, there is very little in the way of discussion: What does it mean? What are you trying to ascertain, how are statistical and systematic errors looking, etc. I think in this section in particular, you'd benefit quite a bit by reading some published, peer-reviewed physics or astronomy papers that you're interested in. You'll see how figures and discussion work. Just as I mentioned above, you'll want to number each figure and have an excellent caption for the reader. Furthermore, your results and discussion text will refer to every figure and comment on them.
From our measured values for the wavelengths, we have:
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =409.84 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =N/A\,\! }[/math]
- [math]\displaystyle{ n=5\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =433.92 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =433.3 nm\,\! }[/math]
- [math]\displaystyle{ n=4\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =485.96 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =485.62 nm\,\! }[/math]
- [math]\displaystyle{ n=3\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =657.4 nm\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =655.9 nm\,\! }[/math]
And according to [3], the accepted values for the four visible wavelengths of hydrogen in the Balmer series are:
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =410.174 nm\,\! }[/math]
- [math]\displaystyle{ n=5\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =434.047 nm\,\! }[/math]
- [math]\displaystyle{ n=4\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =486.133 nm\,\! }[/math]
- [math]\displaystyle{ n=3\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda =656.272 nm\,\! }[/math]
From these values we can calculate our measured Rydberg's constant:
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}), n=3,4,5,6\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{n^2-4}{4n^2})\,\! }[/math]
- [math]\displaystyle{ R=\frac{4n^2}{\lambda(n^2-4)}\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{n^2-4}{4n^2})\,\! }[/math]
- [math]\displaystyle{ \frac{1}{\lambda }=R(\frac{1}{2^2}-\frac{1}{n^2}), n=3,4,5,6\,\! }[/math]
- [math]\displaystyle{ n=6\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =409.84 nm\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(6)^2}{(409.84\times10^{-9} m)((6)^2-4)}\approx1.0979895\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ n=5\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =433.92 nm\,\! }[/math]
- [math]\displaystyle{ R_{Hydrogen}=\frac{4(5)^2}{(433.92\times10^{-9} m)((5)^2-4)}\approx1.0974153\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =433.3 nm\,\! }[/math]
- [math]\displaystyle{ R_{Deuterium}=\frac{4(5)^2}{(433.3\times10^{-9} m)((5)^2-4)}\approx1.0989856\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ n=4\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =485.96 nm\,\! }[/math]
- [math]\displaystyle{ R=\frac{4(4)^2}{(485.96\times10^{-9} m)((4)^2-4)}\approx1.0984840\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =485.62 nm\,\! }[/math]
- [math]\displaystyle{ R=\frac{4(4)^2}{(485.62\times10^{-9} m)((4)^2-4)}\approx1.0982524\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ n=3\rightarrow n=2\,\! }[/math]
- [math]\displaystyle{ \lambda_{Hydrogen} =657.4 nm\,\! }[/math]
- [math]\displaystyle{ R=\frac{4(3)^2}{(657.4\times10^{-9} m)((3)^2-4)}\approx1.0952236\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ \lambda_{Deuterium} =655.9 nm\,\! }[/math]
- [math]\displaystyle{ R=\frac{4(3)^2}{(655.9\times10^{-9} m)((3)^2-4)}\approx1.0977283\times10^7 m^{-1}\,\! }[/math]
The average value of our measured Rydberg's constant can be calculated as:
- [math]\displaystyle{ R_{average}=\frac{1}{n}\sum {R_i} }[/math]
where [math]\displaystyle{ n\,\! }[/math] is the total number of [math]\displaystyle{ R_i\,\! }[/math] So,
- [math]\displaystyle{ R_{Hydrogen,average}=\frac{(1.0979895+1.0974153+1.0984840+1.0952236)\times10^7m^{-1}}{4} }[/math]
- [math]\displaystyle{ =\frac{4.3891124\times10^7 m^{-1}}{4}\,\! }[/math]
- [math]\displaystyle{ \approx1.0972781\pm 0.0025\times10^7 m^{-1}\,\! }[/math]
- [math]\displaystyle{ R_{Deuterium,average}=\frac{(1.0989856+1.0982524+1.0977283)\times10^7m^{-1}}{3} }[/math]
- [math]\displaystyle{ =\frac{3.2949663\times10^7 m^{-1}}{4}\,\! }[/math]
- [math]\displaystyle{ \approx1.0983221\pm 0.0007\times10^7 m^{-1}\,\! }[/math]
The error for our measured value relative to the accepted value is then given by:
- [math]\displaystyle{ \% error=\frac{R_{accepted}-R_{measured}}{R_{accepted}} }[/math]
- [math]\displaystyle{ \% error_{Hydrogen}=\frac{1.0967758\times 10^7 m^{-1}-1.0972781\times10^7 m^{-1}}{1.0967758\times 10^7 m^{-1}} }[/math]
- [math]\displaystyle{ \approx0.046%\,\! }[/math]
- [math]\displaystyle{ \% error_{Deuterium}=\frac{1.0967758\times 10^7 m^{-1}-1.0983221\times10^7 m^{-1}}{1.0967758\times 10^7 m^{-1}} }[/math]
- [math]\displaystyle{ \approx0.141%\,\! }[/math]
- The [math]\displaystyle{ \pm 0.0025\,\! }[/math] and [math]\displaystyle{ \pm 0.0007\,\! }[/math] come from the SEM of the values that we used to calculate the mean.
SJK 18:02, 29 November 2009 (EST)

Unless you have other ideas, I think for your "extra data" session, you should focus on the issue of whether you can discern the difference between H and D with your apparatus (I think the answer is "no"). You can approach the issue statistically (how small could you get the uncertainty with your instrument, and how does this compare with the difference between H and D. This will make your results section longer, and allow you to have more discussion. Also, you can expand your introduction to talk about difference between H and D.
Conclusions
SJK 00:42, 30 November 2009 (EST)

I think you are on the right track for conclusions, but more can be included: "In conclusion, we were able to precisely measure the Rydberg constant for both hydrogen and deuterium. The accepted value for the Rydberg constant for Hydrogen was statistically significantly different from our measurement (___ SEMs discrepancy), indicating some systematic error. Our measurements for H and D were statistically signficantly different, although theoretically their difference should be too small for us to discern with this instrument. Possible reasons for these results are ... and can be investigated further by ...
According to our data, although the Deuterium spectral lines varied from the Hydrogen lines in wavelength, as seen by the percentage error in the Rydberg Constant of each, the variance is slight. The largest value by which the wavelengths varied was designated in the red wavelength measurement as seen in our data tables. But also according to our data, the wavelength measurements of each color seemed to be shifted from the Hydrogen in the Deuterium spectrum.
Acknowledgements
SJK 00:48, 30 November 2009 (EST)

The style of your acknowledgements can be improved for a formal report. "I thank Alexandra S. Andrego for assistance in data collection and analaysis and for useful discussions (ref#xx: a reference that links to her lab). We thank Prof. Gold for use of his lab manual (ref:xx) as a guide for calibration and data collection. We also thank the multiple authors of Wikipedia for useful open-access articles about the Balmer series (refxx) and Deuterium (refxx)." You do not need to acknowledge software that you specifically cite in the methods section (e.g. Google Docs). The same would be true of the Wikipedia articles. But if you'd like to specifically acknowledge particularly useful things, that's OK too. Actually, including references in your acknowledgements like I've suggested is not very typical. But I think it's OK in these circumstances.
Please note that Alexandra S. Andrego was my lab partner for this lab. Her version of this lab can be found here
- Prof. Gold's Lab Manual served as a loose guideline for our lab procedure and our calibration wave lengths
- We used Google Docs to format and post our raw data and error analysis to our wiki notebook
- Our accepted values for the Balmer Series came from hyperphysics.com
- Wikipedia had a great article on the Balmer Series and we used it to confirm our results and understanding for this lab
- Wikepedia 2 is an article on Deuterium
References
SJK 18:04, 29 November 2009 (EST)

References should be numbered, and should be formatted in some standard way (such as author list, title, year, journal, volume, hyperlink). Each journal has its own format, so you can choose from a variety. But just providing a named link is not enough.
Appendix
Derivation of the Rydberg Equation
We can start from the equation of total energy of an electron in the nth energy state derived from the Bohr model:
- [math]\displaystyle{ E_\mathrm{total} = - \frac{m_e e^4}{8 \epsilon_0^2 h^2}. \frac{1}{n^2} \ }[/math]
The change in energy of an electron transitioning from one energy state with a value [math]\displaystyle{ n }[/math] to another is:
- [math]\displaystyle{ \Delta E = \frac{ m_e e^4}{8 \epsilon_0^2 h^2} \left( \frac{1}{n_\mathrm{initial}^2} - \frac{1}{n_\mathrm{final}^2} \right) \ }[/math]
Using [math]\displaystyle{ \frac{1}{ \lambda} = \frac {E}{hc} \rightarrow \Delta{E} = hc \Delta \frac{1}{\lambda}\,\! }[/math] to change the units to wavelength, we get
- [math]\displaystyle{ \Delta \left( \frac{1}{ \lambda}\right) = \frac{ m_e e^4}{8 \epsilon_0^2 h^3 c} \left( \frac{1}{n_\mathrm{initial}^2} - \frac{1}{n_\mathrm{final}^2} \right) \ }[/math]
where
- [math]\displaystyle{ h \ }[/math] is Planck's constant,
- [math]\displaystyle{ m_e \ }[/math] is the rest mass of the electron,
- [math]\displaystyle{ e \ }[/math] is the elementary charge,
- [math]\displaystyle{ c \ }[/math] is the speed of light in vacuum, and
- [math]\displaystyle{ \epsilon_0 \ }[/math] is the permittivity of free space.
And the Rydberg constant for Hydrogen is found as:
- [math]\displaystyle{ R_H=\frac{m_e e^4}{8 \epsilon_0^2 h^2}\,\! }[/math]
List of Used Constants
- [math]\displaystyle{ \mu\,\! }[/math] is the reduced mass of an atom
- [math]\displaystyle{ e=1.602\times10^{-19} C\,\! }[/math]
- [math]\displaystyle{ \epsilon_0=8.854\times10^{-12} F\cdot m^{-1}\,\! }[/math]
- [math]\displaystyle{ c=2.998\times10^8 m\cdot s^{-1}\,\! }[/math]
- [math]\displaystyle{ h=6.626\times10^{-34}J\cdot s\,\! }[/math]
- Rydberg's constant for hydrogen is calculated to be approximately:
- [math]\displaystyle{ R\simeq1.0967758\times10^7m^{-1}\,\! }[/math]