Physics307L F09:People/Gleicher/Planck
Planck's Constant Lab Summary
Abstract
Classically, electro-magnetic radiation was viewed as continuous waves of energy. The energy of the radiation is related to the intensity of the incident light and the intensity is proportional to the time-averaged square of the electric field. Accordingly, when a source of constant power illuminates an object for a period of time the imparted energy is dependent on the time of exposure.
Classical theory predicts that the amount of light emitted from a blackbody would increase as wavelength decreased but experiment showed that it decreased with wavelength. This is known as the "ultraviolet catastrophe" [1](under lab manual).
Using a theory of quantized or "bundled" light energy, Max Planck (1858-1947) was initially able to reconcile the disagreement between theory and experiment regarding the emission from a blackbody radiator. In a paper originally submitted by Planck in December 1900, Planck proposed that the energy of a photon was related to the frequency by a factor of h, or Planck's constant:
[math]\displaystyle{ h*\nu=E }[/math],
Planck was awarded the Nobel Prize in 1918 for his theory of quantized EM radiation. [2]
Albert Einstein (1879-1955) later used the photon concept to describe the photoelectric effect, wherein a photon impinging on a clean metal surface frees an electron from the metal. His theory stated that if a photon had more energy than the work function of a metal, then an ejected electron would be freed with a kinetic energy equal to the difference in energies.
[math]\displaystyle{ Etot=KE+\phi }[/math]
Einstein won the Nobel Prize in 1921 for his theory of the photoelectric effect.
Setup
SJK 00:27, 19 November 2007 (CST)

OK, these comments will be for your "Lab 3 grade" as opposed to the formal report page. You should record the model numbers of all the equipment you are using (in this case, a h/e apparatus, and voltmeter, anything else?
Using a mercury vapor lamp as a light source, the light is split by a transmission grating into its constituant lines. The different lines with varying frequencies can be focused onto our apparatus. The apparatus is described in the lab manual[3]. The outside of the apparatus has a white mask through which our light can be shone. Inside the apparatus is a metal with an applied reverse bias. The apparatus has an op-amp circuit with very high impedance which allows the measurement of the voltage without draining the charging current.
If the energy of the impinging photons is greater than the work function than a current of photo-electrons is created. When a reverse biased voltage is applied, the current is reduced. If the voltage when the most energetic electrons are stopped is known, then the maximum kinetic energy of the electrons can be calculated.
Graphing the stopping voltage versus the frequency of the light will allow the extraction of the work function Wo and h from a least squares fit.
For the first part of the experiment we timed how long it would take to reach a given stopping voltage for a given line while varying the amount of light that could get through a blocking filter.
The point of the first part is to test the classical theory of EM radiation, and to determine if the energy imparted increases with time.
For the second section of the lab I try to experimentally determine the value of Planck's constant and the work function. After taking several sets of data I can plot the stopping voltage vs. wavelength and by analyzing the data I hope to end up with a value for Planck's constant close to the accepted value of 6.626068E-34 J*s.
Procedure
The first section of the experiment was performed in the following manner:
1) The lines were sequentially focused on the apparatus mask, starting at one end of the spectrum. There were five lines in the resulting orders. They were in order of yellow, green, blue-green, blue, and blue-violet. Measuring the yellow and green lines required the use of spectral filters in front of the grating to prevent higher energy photons from hitting the apparatus.
2) The stopping voltage is measured while timing how long it takes to reach approximately 90% of the maximum voltage.
3) Using the yellow and green lines, the intensity of the light was varied using a variable transmission filter to determine the effect of intensity on the energy transmitted.
The second section, determination of h, involved measuring the stopping voltages of all five lines. The stopping voltage plotted against the frequency will allow calculation of the work function, [math]\displaystyle{ \phi }[/math], and the value of h. Using several sets of data will increase the accuracy of the calculation.
Results
The data for this experiment can be found here: Physics307L_F09:People/Gleicher/Notebook/071008
Section I
Blue Line:
| Percentage | Trial 1 (s) | Trial 2 (s) |
|---|---|---|
| 100 | 1.09 | 1.78 |
| 80 | 1.66 | 2.34 |
| 60 | 2.65 | 2.30 |
| 40 | 3.94 | 2.56 |
| 20 | 4.72 | 5.81 |
Green Line:
| Percentage | Trial 1 (s) | Trial 2 (s) |
|---|---|---|
| 100 | 3.22 | 3.19 |
| 80 | 3.34 | 4.11 |
| 60 | 5.96 | 8.72 |
| 40 | 9.50 | 12.28 |
| 20 | 16.72 | 18.4 |
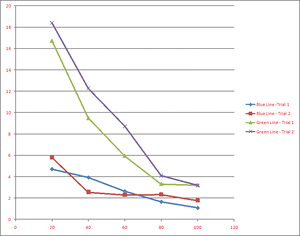
The charging time vs transmission is plotted in the chart to the right. It is apparent that increasing the transmission reduces the charging time. This can be explained by the photon theory in the following way. The photons all have the same energy but the filter reduces the amount of photons, which reduces the photocurrent, which causes the charging of the circuit to take longer.
The stopping voltage for the blue line was 9.49 V while the stopping voltage for the green line was less. This makes sense if the frequency of the light is proportional to the energy of the light, since the blue light has a higher frequency than the green light.
Even though the rate at which the voltage accumulated was different for the varying transmissions, the final stopping voltages did not change.
I do not believe that this result supports the classical theory due to the fact that the stopping voltage did eventually reach a maximum, despite the fact that the charging times were indeed different.
Section II
For this section, the stopping voltages for five different lines were measured.
| Line | Vstop - Trial 1 | Vstop - Trial 2 |
|---|---|---|
| Yellow | 0.723 V | 0.722 V |
| Green | 0.856 V | 0.853 V |
| Blue/Green | 1.501 V | 1.501 V |
| Blue | 1.716 V | 1.713 V |
| Blue/Violet | 2.063 V | 2.064 V |
As one can see, as the wavelength decreases the stopping voltage increases, hence the electron energy increases.
The results of my experiment support the quantum model of light. The intensity of the light did not have a real pronounced effect on the stopping voltage, whereas varying the wavelength of incident light significantly changed the stopping voltage.
At first my results for the first part of the experiment seem to support the classical theory such that the stopping voltage did vary with time, until it reached the point at which the current stopped. It is at that point that the stopping voltage becomes constant. This suggests that it indeeds takes time to reach the stopping voltage, but the final value does not increase with time. Whereas when the frequency of the impinging light is varied the stopping voltage is proportional to the frequency.
Determination of h
By plotting several measurements from the first and second orders I hope to determine the value of h and the work function Wo.
This is the data that was taken:
First Order
| Line | Set 1 | Set 2 | Set 3 | Set 4 |
|---|---|---|---|---|
| Yellow | 0.716 | 0.723 | 0.721 | 0.722 |
| Green | 0.856 | 0.854 | 0.853 | 0.854 |
| Blue/Green | 1.502 | 1.501 | 1.516 | 1.515 |
| Blue | 1.71 | 1.718 | 1.712 | 1.716 |
| Blue/Violet | 2.07 | 2.069 | 2.071 | 2.071 |
Second Order
| Line | Set 1 | Set 2 | Set 3 | Set 4 |
|---|---|---|---|---|
| Yellow | 0.773 | 0.764 | 0.768 | 0.770 |
| Green | 1.255 | 1.268 | 1.260 | 1.263 |
| Blue/Green | 1.557 | 1.559 | 1.557 | 1.558 |
| Blue | 1.734 | 1.728 | 1.730 | 1.731 |
| Blue/Violet | 2.066 | 2.061 | 2.064 | 2.062 |
This is a link to my excel file with the calculations for Wo and h.
My values for the first and second order are as follows:
SJK 00:32, 19 November 2007 (CST)

You don't have units on these numbers! That is a serious problem. Less serious is that you have too many digits. It would be better to write, for example: (1.17 +/- 0.15) E-33 J-s
First Order
h = (1.17817 +/- 0.154653)E-33
Wo = (-4.95859 +/- 0.94522)E-19
Second Order
h = (9.98274 +/- 1.54485)E-34
Wo = (3.70127 +/- .944192)E-19
SJK 00:33, 19 November 2007 (CST)

Even for an informal lab summary, you still need to discuss your final results, and compare with the accepted value, etc.