Physics307L:People/Wilkinson/Electron
Electron Diffraction Lab Summary
Steve Koch 01:05, 22 December 2010 (EST):Great summary and I like the further exploration of the systematic error / correction!
Purpose
The purpose of this lab was to experimentally confirm the wave nature of classical particles (electrons). To do this we accelerated electrons in a potential and passed them through a crystal lattice (graphite) of known d-spacing. We then observed the resulting diffraction maxima that appeared on a screen several centimeters away. We measured the diameter of these maxima and then varied the accelerating potential, taking more measurements of the resulting diameters. With this data we were able to show a linear relationship between the accelerating potential (ie. the kinetic energy the electrons gained) and the diameter of the diffraction maxima. We were also able to use this data to estimate the d-spacing of the graphite lattice to within 8% of the expected value.
Data
We estimate the d-spacing of the crystal lattice to be d = .195E-9 ± 1E-12 m and d = .114E-9 ± 0.4E-12 m where the accepted values are [math]\displaystyle{ d = 0.213*10^{-9} m }[/math] and [math]\displaystyle{ d = 0.213*10^{-9} m }[/math]. Generating an 8% and 7% error respectively. Since our standard error is not in the range of where the accepted value lies there must be some standard error associatied with our measurments. We believe that this error is a function of not being able to accurately measure the accelerating voltage, human error in measurment of the peaks, and optical problems in the peak display on the glass.
We were also able to generate linear plots of diameter vs. 1/sqrt(kV).
Data can be viewed in my lab notebook Lab Data
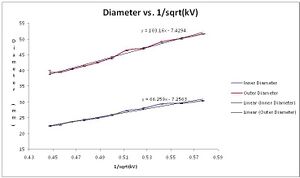
Corrected Data
After reporting our results we realized that the measurement of the diffraction patter rings was made incorrectly. See Lab Data for further explination. Our new values for the d-spacing are d = .212(1) nm and d = .1201(5) nm. These new values have a 1% and 2% error respectively. There still may be some standard error because the second d-spacing calculated value doesn't fall within 1 SEM.
History
The fact that photons, electrons, and other small physical entities can behave as both particles and waves is a relatively new idea in the physics community. For example, light is classically characterized as a wave of electromagnetic radiation. Though this characterization was found to be wanting when it came to describing the photoelectric effect. Light had to act, almost simultaneously, as both a particle and a wave! Louis de Broglie took this deviation from logic one step further when he asserted that classical particles could act like waves. It is this assertion that we put to the test.