Physics307L:People/Trujillo/Planck's
Plank's Constant
SJK 00:15, 10 December 2007 (CST)

The link to the file is fine, I was able to view it. I think you are using LINEST with the arguments reversed (the first argument is the y's , then the x's)...but there is also a problem where LINEST doesn't like tiny numbers. In any case, I think this is totally a symptom of not enough time to work things out!
Purpose
In this lab we are using a Hg vapor mechanism to determine planks constant. In experiment 1 we determined the stopping voltage for 4 colors of light on the light spectrum. We should be able to find a relationship between light energy and the frequency of the incident light. With the second experiment we are to determine the constant of proportionality ([math]\displaystyle{ h }[/math])
Data
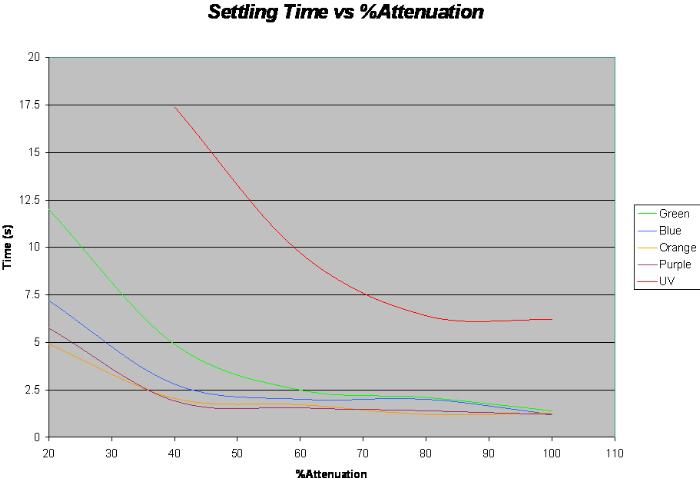
Instead of using a stop watch to catch the settling voltage, we used a scope. First we measured the the max photo electric voltage for each color individually, then determined 90% value of that voltage. We acquired the voltage and used the x and y cursers to determine the time it took to get to the %90 value recorded. This method was much more accurate but it took a bit longer to take the data
| % Attenuation | Orange | Green | Blue | Purple | UV |
|---|---|---|---|---|---|
| 100 | 1.28 | 1.4 | 12 | 1.2 | 6.2 |
| 80 | 1.2 | 2.1 | 2 | 1.4 | 6.4 |
| 60 | 1.72 | 2.5 | 2 | 1.56 | 9.7 |
| 40 | 2.04 | 4.9 | 2.8 | 1.92 | 17.4 |
| 20 | 4.92 | 12 | 7.2 | 5.76 | 81 |
| Orange | Green | Blue | Purple | UV | |
|---|---|---|---|---|---|
| Stopping Voltage | 652mV | 800mV | 1.37V | 1.50V | 1.80V |
| 90% | 612mV | 712mV | 1.23 | 1.34 | 1.62 |
SJK 00:16, 10 December 2007 (CST)

Nice plot, what does it mean?
Observations
I played around with the the line fitting and could not get an acceptable value for h (2.7654E-15). See the attached .xls file. I used LINEST to use the least squares approach to finding h. The instructions in Golds lab manual was bit unclear on how exactly to calculate h. As far as the physical observations, It was clear that different color of light gave of a different photo electric effect and of course when the attenuation lenses were applied, the effect was attenuated by certain degree. I was unable to see the photoelectrons oscillate at a certain frequency. I would like to see the equation that proves that the kinetic energy of a photoelectron is proportional to the frequency of its incident light. Media:Plank.xls --I dont know how else to link to a file.