Physics307L:People/Josey/Excitation and Ionization Levels of Neon
Steve Koch 02:47, 21 December 2010 (EST): Obviously my feedback way too late, but as I said in email back then, I think you misinterpreted what the "peaks" are, due to the negative values. Very nice data taking and notebook, though.
Excitation and Ionization Levels of Neon
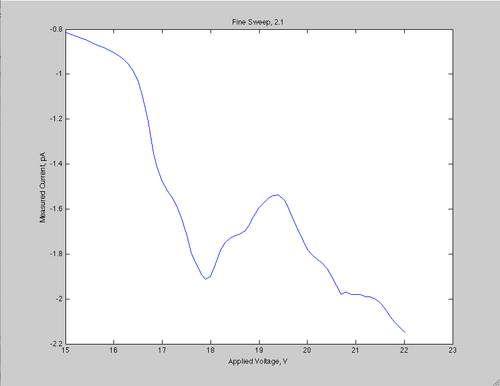
This week, my partner, Kirstin, and I measured the ionization and excitation levels of neon. To do this we bombarded neon atoms with electrons of a given energy level, and measured the resulting current across a ring in the chamber containing the neon. To bestow the energy on to the electrons, we gave them a certain potential, and let them strike the neon. The neon would then either lose an electron, excite and then lose energy, or nothing from the collision. This would be detected by the current reading, and then plotting the current as a function of the voltage would then produce a graph. Here is a sample graph:
This graph is the plot of a set of data we got by setting the Vf to 2.1, and raising the applied voltage on the electrons from 15 V to 22 V in 0.1 V increments. As you can see there are some prominent peaks. These peaks are the result of neon atom being excited by the collision with the electron, and then returning to its ground state. Then around 21V, the current levels off for a while, and then begins to fall again. This is the result of the ionization, where the electron knocks an atomic electron free.
Results
As explained in more detail in my notebook, we were able to measure two excitation levels and the ionization level. From our data the excitation levels were:
- 16.5 ± 0.01 eV
- 19.46±.3 eV
We know the accepted values are 16.7eV and 18.65eV. Because of the discrepancy in our data from the accepted value, it is clear that there is some source of error in our measurements. The major issue we came across was determining the peaks from the data. To find them, we would look at the plots of our data, and find the places at which there would be peaks, and then pour over the data to find them. In the case of the first excitation level, the 16.5 eV, the peak was very slight. However, were were more fortunate when measuring the ionization level:
- 21.6 ± 0.2 eV
This value is close enough to the accepted value, 21.56 eV, that it is clear we were able to successfully measure the ionization level. This value was much clearer to see, as it is the point where the current levels out for a short period of time, and then begins to fall again as more electrons and knocked out of the upper orbits of the neon atoms. However, after setting up the apparatus, this experiment was fairly simple and easy to run.