Particle Surface Interactions in Flow - Jocelyn Yuen and Davis Miller
Introduction
The study of particle surface interactions, in terms of controlling the selective adhesion of specific molecules, has been an ongoing interest in the science field such as for tissue scaffolds, sensors, smart adhesives, and separation media[1]. A general trend that has been used to control these interfacial properties has been to use surface modifications, or surface fabrications, inspired by nanopatterns[2][3] and chemical cues in biology[4][5] to adjust the selective adhesion of certain particles. However, a lack of emphasis on adhesion rate in cohesion with selectivity has yet to be studied[6]. A larger focus to consider not only the chemical properties of surfaces but also the physical mechanisms of deposition are used to understand and optimize control over adhesion and selectivity. These objectives are used to explore the kinds of surface modifications that are necessary in order to attain the specific particle-surface interactions relevant to a particular application.
The simplest of devices to test particle surface interactions in flow is a parallel plate flow chamber. Typically, a rectangular flow chamber, with an inlet and outlet port, is fabricated with specific dimensions; when testing, a glass slide is placed on top to create the surface. This device allows for controlled shear flow to study the system and conditions desired. This may be further applied in microfluidics.
Microfluidics provides the perfect tools for further understanding the behavior of these nanoscopic features effecting dynamic adhesion of particles onto surfaces. Numerous surface studies have successfully used microfluidic network systems to implement and model surface specific[3][5]. The technology and devices used in microfabrication allow one to achieve large shear stresses while still maintaining laminar flow, providing a wider range of experimental conditions which can not otherwise be tested at a larger scale. Another benefit of transitioning this study into microfluidics is that it allows one to fabricate more complex designs which can creatively model more complex systems such as microvascular networks[21].
Adhesion Factors
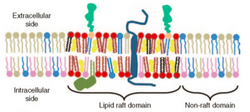
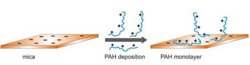
In general, the specific surface chemistry and surface physics in each particle adhesion situation are dependent on the application of study.
For biological systems, particle surface studies are focused on cell membrane adhesion which is based on functions of surface concentrations and surface densities of ligands, receptors, or peptides[3]. In vivo, these adhesive groups often move over time and space causing chemical and topographical heterogeneities of the surface. For example, lipid rafts within the cell membrane are used to promote receptor accumulation to a localized area. The accumulation of these receptors by this phenomena changes the spatial density and functionality of the membrane, either inducing or hampering proteins binding to the surface[8]. The orientation of these adhesive groups are highly specific and required in order to attain cell adhesion. Thus, a major challenge to engineering biomimetic surfaces has been finding ways to achieve these definitive micron-scale patterns to reproduce the same selective in vivo cell surface interactions [3].
As colloids often carry an electrical charge, electrostatic heterogeneities play a large implication in adhesion for these applications. These interactions are dependent on electrostatic forces in combination with van der Waals forces which have been modeled by the DVLO (Deryaguin, Landau, Verwey, and Overbeek) theory and has been discussed by Holt et al. [11]. Studies have shown to use manipulation of the pH and ionic strength of colloid suspensions, as well as the concentrations of added surfactants to control particle adsorption onto polyelectrolyte surfaces [14]. Similar to biological applications, these electrokinetic characteristics of polyelectrolytes have been shown to deposit in a "patchy" manner. An example can be seen in geology and mineral extraction such as where cationic monolayers on mica have been shown to form heterogeneous and nonuniform surfaces naturally[12].
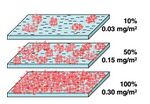

Particle Surface Adhesion in Flow
There are two main categories of forces affecting particle adhesion in flow, those being hydrodynamic forces and physicochemical forces.
Physicochemical forces include forces arising from hydrogen bonding, electrostatic forces (charge differences), hydrophobicity/hydropholicity, steric forces, and Van der Waals forces. In practice many if not all of these forces are present, making it difficult to predict particle behavior. However, in controlled conditions desired specifications may be applied, allowing for controlled particle transfer. Essentially, the relative size of all of these forces between the surface and particle result in the adherence or repelling of the particle.
Hydrodynamic forces are those that arise from flow, such as drag. These forces play an interesting role in particle capture, as they may hinder or promote adhesion. Increased flows, and therefore increased hydrodynamic forces, would hinder particle capture by outweighing physicochemical interactions and pushing particles away from the surface. On the contrary, increased flow in turn increases the mass transfer coefficent between the particle and surface, thereby aiding capture[2]. S. Kalasin and M. Santore investigated these phenomena in a silica surface modified with cationic polymer patches (Figure 3), and found what is known as the hydrodynamic crossover. The results may be found in Figure 4. It is seen that this crossover is dependent on the number of sites there are for particles to adhere (surface coverage). Below a certain critical surface coverage, flow hinders adhesion as there is not enough interaction from the surface. Beyond that point, however, increased flow aids transfer and adhesion[2].
To summarize, a list of some factors which may be considered when looking at particle surface deposition include:
- hydrodynamic forces of the particles
- particle size and shape
- chemical and topographical surface features
- concentration or thickness layer of the material deposited onto the surface
- electrostatic attractions and colloidal forces between the two.
Surface Modification Techniques
Photolithographic methods
Physical barriers created by photolithography have been used as means to modify surfaces . This allows for the controlled deposition and attachment of materials such as biomolecules. However, this is typically done with planar surfaces and difficult to achieve with curved surfaces[5].
Micro-Contact Printing Techniques
Microcontact printing is a form of soft-lithography that utilizes patterns created on a PDMS master to "print" self-assembled monolayers of ink on a surface. The ink is diffused into the PDMS master, and when brought into contact with the surface, the solution is transferred, retaining its pattern. However, this process is limited by the resolution of the PDMS stamp in addition to the transfer efficiency of the ink agent[5].
Polymer Brushes
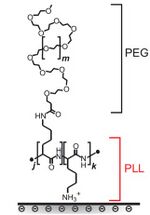
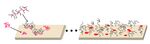
Many surfaces modifications are done by adding what's known as a polymer brush. These brushes consist of a graft copolymer, meaning a copolymer with a backbone of polymer A and sidechains of polymer B. Typically, the brush is made of a poly(L-lysine) (PLL) backbone and poly(ethylene glycol) (PEG) side chains. PLL is cationic, so it may bind to surfaces such as negative silica, whereas PEG is neutral, hydrophilic, and capable of hydrogen bonding. This gives the brush protein-repellent properties[15]. The chemical structure of a PLL-PEG brush is found in Figure 5.
To make a polymer brush surface, a buffer (usually phosphate buffer) is flowed over the unmodified surface to equilibrate. Then, the same buffer but containing the polymer is flowed, allowing the brushes to form. Finally, buffer without polymer is flowed to remove any non-adhered polymer. In the case of PLL-PEG, the PLL tethers to the surface, allowing the PEG side chains to stick up like a brush[19]. This may be seen in Figure 6.
Surfaces may consist of various other modifications, such as with polymer patches or nanoparticle coatings.
Analyzing Surface Modifications
Atomic Force Microscopy
Atomic force microscopy (AFM) is a type of scanning probe microscopy (SPM) that can achieve extremely high resolutions: on the order of fractions of a nanometer. This method maps the surface by physical interaction of a tip, which is usually on the scale of 10's of nanometers in radius. By measuring the atomic forces between the surface and tip, and employing a laser on the probe while measuring deflection, a feedback loop may be employed to keep this force constant. When the sample height changes, so too does the deflection and force. The feedback loop returns the force to a set value and the deflection is measured, ultimately creating a topographical map[24].
Total Internal Reflection Fluorescence
Total internal reflection fluorescence (TIRF) is an optical technique that allows the study of macromolecule interactions with solid surfaces, such as cells and a glass slide[23]. It can be used to study adsorption in both stagnant and flowing systems to obtain adsorption rates and equilibrium data. Essentially, TIRF works when an incidental beam of light on an interface of two transparent media is totally internally reflected, which depends on the indices of refraction of the two media. This creates an evanescent wave at the point of reflection penetrating into the medium of lower refractive index. An evanescent wave is a stationary electromagnetic wave that decays exponentially with depth. This allows for the design of a wave that penetrates entirely into the adsorbed macromolecule but not into the bulk solution, allowing for the detection of these molecules, provided they can fluoresce at the incident wavelength. The fluorescent intensities may then be correlated to particle concentration[23].
Challenges
There are two main challenges that current work faces. First of all, it is hard to predict all physicochemical interactions and accurately control them. Additionally, when adding surface modifications, there often will be flaws present, such as with polymer brushes there will be patches with no brush or smaller brushes. This in turn may allow, for example, particles to adhere to these flaws when the surface is meant to repel the particles.
Applications of Particle-Surface Interactions
Cell behavior on functionalized surfaces
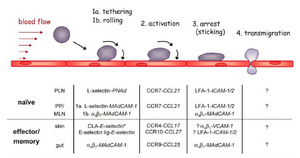
Consider Leukocyte Extravasation as an example where the accumulation of flowing white blood cells in blood vessels are recruited near sites of damaged tissue. The series of behaviors of which these cells interact with the inner surface of the vessels are as follows and is depicted in Figure 7:
As the cells flowing near the functionalized surface [21]:
1. The leukocyte experiences transient tethering and rolling behavior upon first interactions with the inner wall
2. Rolling behavior begins activation of certain integrins prepping its rest phase in stage 3
3. The cells stop rolling and firmly begin to adhere to the inner surface of the wall
4. Lastly, the cells transmigrate through the walls of the vessels into the surrounding infected tissues
The interactions between the cell and inner wall of the vessels depend on the chemical signal of the functionalized surface at each stage. Interesting studies have also been implemented to further understand the hydrodynamic forces of the flowing cells near the surface of which either aid in adhesion or compete with the attraction forces of the functionalized surfaces [19]. In this application, it is curious to understand under what flowing conditions does this affect situations when the cell experiences rolling behavior and when cells adhere firmly to the inner surface wall.
An example of examining the rolling behavior of cells in microfluidics is provided in the video below:[1]
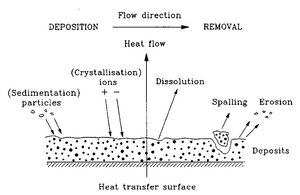
Fouling of materials and devices
More industrial applications include understanding fouling of certain materials and devices in which usually cause depletion in the functional properties of such devices. For example, an accumulation of many substances onto the heat exchanges, such as particulates, sediments, and layers from the result of corrosion, can reduce the efficiency of its work [22]. In addition, fouling can be found commonly in the water treatment industry where microorganisms tend to accumulate on water filters. Biofouling also occurs in biomedical devices and implants where proteins and other non-selective cells adhere to the surfaces of these materials embedded inside the body. The accumulation of these macroparticles often lead to issues of plaque buildup and cause blood clots.
Often the interest in these applications is to develop and use antifouling agents which reduce the deposition of such unwanted particles on the respective surfaces. This helps in maintaining the materials and these devices, allowing them to work more effectively and be more durable long-term.
References
1. Kozlova, N.; Santore, MM. Manipulation of Micrometer-Scale Adhesion by Tuning Nanometer-Scale Surface Features. Langmuir, 22, 1135-1142, (2006). http://dx.doi.org/10.1021/la0515221
2. Frisbie, CD.; Rozsnyai, LF.; Noy, A.; Wrighton, MS.; Lieber, CM. Functional Group Imaging by Chemical Force Microscopy. Science, 265, 2071-2074, (1994). http://dx.doi.org/10.1126/science.265.5181.2071
3. Patel, N.; Padera, R.; Sanders, GHW.; Cannizzaro, SM.; Davies, MC.; Langer, R.; Roberts, CJ.; Tendler, SJB.; Williams, PM.; Shakesheff, KM. Spatially controlled cell engineering on biodegradable polymer surfaces. The FASEB Journal, 12, 1447-1454 (1998).
4. Wu, H.; Henzie, J.; Lin, W.; Rhodes, C.; Li, Z.; Sartorel, E.; Thorner, J.; Yang, P.; Groves, JT. Membrane-protein binding measured with solution-phase plasmonic nanocube sensors. Nature Methods, 9, 1189-1191 (2012). http://dx.doi.org/10.1038/nmeth.2211
5. Mansfield, E.; Ross, EE.; D'Ambruoso, GD.; Keogh, JP.; Huang, Y.; Aspinwall, CA. Fabrication and characterization of spatially-defined, multiple component, chemically-functionalized domains in enclosed silica channels using cross-linked phospholipid membranes. Langmuir, 23, 11326-11333 (2007). http://dx.doi.org/10.1021/la7008946
6. Santore, MM.; Kozlova, N. Micrometer Scale Adhesion on Nanometer-Scale Patchy Surfaces: Adhesion Rates, Adhesion Thresholds, and Curvature-Based Selectivity. Langmuir, 33, 4782-4791 (2007). http://dx.doi.org/10.1021/la063546t
7. Sergent, O.; Aliche-Djoudi, F.; Lagadic-Gossmann, D. Up-to-Date Insight About Membrane Remodeling as a Mechanism of Action for Ethanol-Induced Liver Toxicity. Trends in Alchoholic Liver Disease Research - Clinical and Scientific Aspects (2012). http://dx.doi.org/10.5772/27410
8. Studying the Role of Lipid Rafts on Protein Receptor Bindings with Cellular Automata. IEEE/ACM Transactions on Computational Biology and Bioinformatics, 10, 760-770, (2013). http://dx.doi.org/10.1109/TCBB.2013.40
9. El-Sayed, A.; Harashima, H. Endocytosis of Gene Delivery Vectors: From Clathrin-dependent to Lipid Raft-mediated Endocytosis. Molecular Therapy, 21, 1118-1130 (2013). http://dx.doi.org/10.1038/mt.2013.54
10. Israelachvili, JN.; Adams, GE. Measurement of forces between two mica surfaces in aqueous electrolyte solutions in the range 0–100 nm. Journal of the Chemical Society, Faraday Transactions 1, 74, 975-1001 (1978). http://dx.doi.org/10.1039/F19787400975
11. Holt, WJC.; Chan, DYC. Pair Interactions between Heterogeneous Spheres. Langmuir, 13, 1577-1586 (1997). http://dx.doi.org/S0743-7463(96)00389-7
12. Nishimura, S.; Scales, PJ.; Biggs, SR.; Healy, TW. AFM studies of amine surfactant hemimicelle structures at the mica-water interface. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 103, 289-298 (1995). https://doi.org/10.1016/0927-7757(95)03256-D
13. Morga, M.; Adamczyk, Z. Monolayers of cationic polyelectrolytes on mica – Electrokinetic studies. Journal of Colloid and Interface Science, 407, 196-204 (2013). http://doi.org/10.1016/j.jcis.2013.05.069
14. Chen, KM.; Jiang, XP.; Kimerling, LC.; Hammond, PT. Selective self-organization of colloids on patterned polyelectrolyte templates. Langmuir, 20, 7825-7834 (2000). http://dx.doi.org/10.1021/la000277c
15. Gon, S.; Kumar, KN.; Nusslein, K.; Santore, MM. How Bacteria Adhere to Brushy PEG Surfaces: Clinging to Flaws and Compressing the Brush. Macromolecules, 45, 8373-8381 (2012).
16. Kalasin, S.; Santore, MM. Hydrodynamic Crossover in Dynamic Microparticle Adhesion on Surfaces of Controlled Nanoscale Heterogeneity. Langmuir, 24, 4435-4438 (2008).
17."Single Component and Selective Competitive Protein Adsorption in a Patchy Polymer Brush: Opposition between Steric Repulsions and Electrostatic Attractions " S. Gon and M.M. Santore, Langmuir, 27, 1487-1493. (2011).
18.“Engineering Nanoscale Surface Features to Sustain Microparticle Rolling in Flow” S. Kalasin and M. M. Santore, ACS Nano 9(5), 4706-4716 (2015).
19.“Near-Surface Motion and Dynamic Adhesion during Silica Microparticle Capture on a Polymer (Solvated PEG) Brush via Hydrogen Bonding” S. Kalasin, and M. M. Santore, Macromolecules 49(1) 3334-343 (2016).
20.Röttgermann, P. J. et al. Cell motility on polyethylene glycol block copolymers correlates to fibronectin surface adsorption. Macromolecular bioscience, 14(12), 1755-1763. (2014).
21.Doshi, N.; Prabhakarpandian, B.; Rea-Ramsey, A.; Pant, K., Sundaram, S.; & Mitragotri, S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. Journal of Controlled Release. 2010, 146(2), 196-200
22. S. N. Kazi (2012). Fouling and Fouling Mitigation on Heat Exchanger Surfaces, Heat Exchangers - Basics Design Applications, Dr. Jovan Mitrovic (Ed.), InTech, DOI: 10.5772/32990.
23. Lok, B. K., Cheng, Y. L., & Robertson, C. R. (1983). Total internal reflection fluorescence: A technique for examining interactions of macromolecules with solid surfaces. Journal of Colloid and Interface Science, 91(1), 87-103. https://doi.org/10.1016/0021-9797(83)90316-8
24. How an AFM Works. Nanoscience Intsruments, 2017 (accessed Apr 21, 2017).
