Microfluidic Sensing- Nick Uvanovic
Microfluidic Sensing
Microfluidics have enabled a new generation of micro sensing, with a vast range of applications across countless industries. Sensor development and integration in microfluidic devices have become a vocal point in microfluidic research, with optical, and electro-based methods, generally considered the 2 major types of sensing coupled with microfluidics. However, it should be noted that sensing technologies in microfluidics are rapidly evolving to meet industry applications 1.
Optical Sensing
There are typically a wide variety of micro-scale optical detection sensors that can be integrated into microfluidic systems. Optical sensing is generally considered to be one of the more favored microfluidic detection methods due to its selectivity and sensitivity. Optical detection methods in microfluidics rely on monitoring light properties such upon fluorescence, absorbance, luminescence, and surface plasmon based methods. Most optical sensing methods incorporate integrated electronic processing, such as microprocessors, resonance filtering, fiber optics etc4.
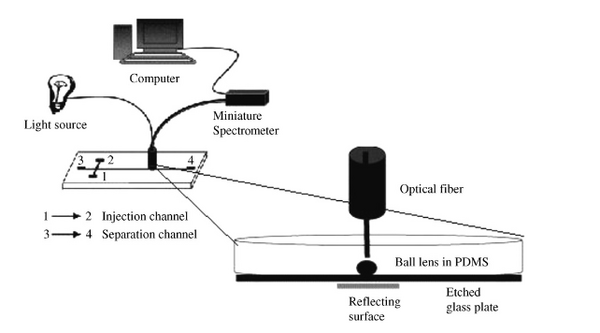
Seen in Figure 1 is an optical absorbance sensing design used for the detection of calcium ions in urine. This system utilizes fiber optics, and a ball lens to couple absorptions signals from a microchip. The system compares absorption and reflectivity measurements at a detection point and is able to measure the presence of calcium ions in the channel 2.

Figure 2 provides a comprehensive clarification into pros/cons of different optical sensing methods commonly seen in microfluidics.
The video above explains the potential of optical microcavities as sensors in medical diagnostics 6.
Fluorescence detection is the most favored and utilized optical sensing method in microsystems due to its superior accuracy and mobility. LIF (laser induced fluorescence) is a spectroscopic method that is most commonly used in microfluidics because it is easily adapted to microdevice dimensions and is a relatively straightforward process. In this method, laser light is used to excite molecules to a higher energy level, allowing for visual observation that would not be possible otherwise. Laser based detection allows for easy focus on small detection volumes, while managing high irradiance levels. The setup needed for LIF is relatively inexpensive and again favored due to its mobility and history of high detection success 3.
In contrast to absorbance sensing, where the sensitivity is limited per Beer's Law, fluorescence detection can be much more mobile. Beer's Law relates the absorption of light to the material through it travels. For very thin samples, as in some microfluidic devices, the absorption of light will not be significant and will therefore limit the detection. However, with sensing methods like LIF the attenuation of light does not define the sensitivity of detection. Fluorescence based methods are typically much more sensitive to the detection of concentration variations. Of course, fluorescence and absorbance based methods both have unique advantages to their sensing attributes, and the application will most likely define which sensing method is used.
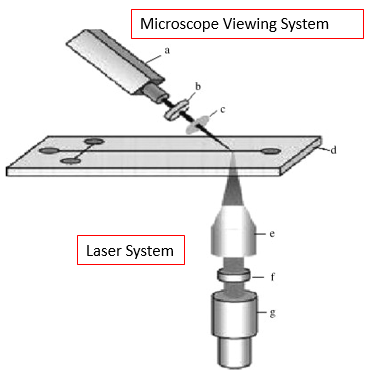
In Figure 3, we can see what a typical LIF setup would look like, where a microdevice is exposed to laser light and observed optically through a microscope system. LIF is used for a wide variety of applications, with one example being detection and characterization of bio-molecules for protein analysis.
Another application of optical sensing in microfluidics utilizes Raman spectroscopy, a technique used to observe low frequency modes in a system. Like LIF, it is also a laser-based detection technique. It is based on Raman scattering, the scattering of photons in molecules that are excited to higher energy. As Raman spectroscopy is typically considered to be limited in intensity, and therefore limited in detection ability, Surface-Enhanced Raman Spectroscopy (SERS) is a more common tool used throughout the scientific community and in microfluidics. Typically, the sample to be studied is enhanced with gold, silver, or other metallic nanoparticles, vastly improving the intensity of signal achieved 8.
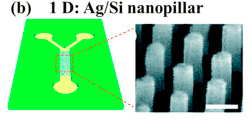
Recent research into SERS based microfluidics has resulted in novel SERS-active substrates that provide a much more consistent Raman signal than colloid substrates. An example of this is a microfluidic device that incorporated Ag coated Si nanopillars into the channel, seen in Figure 3. This device produced highly efficient, highly replicable SERS signals and was used to for identification of double stranded DNA 8.
Lastly, a more recent optical sensing technique employed in microfluidics is the idea of an integrated “lab on a chip” with built in detectors.
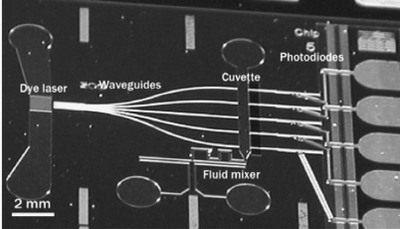
Seen in Figure 5 is a highly integrated microfluidic device composed of individual functional components. The device incorporates a liquid dye laser, waveguides, fluidic channels, measurement cuvettes, and photodiodes all embedded in silicon substrate. The dye laser is coupled into five different waveguides, which brings the light to corresponding fluidic channels for absorbance measurement. The light is then detected by photodiodes and the end of the device. This device was used to for detection of ammonia in pure water, and results proved the successful integration of the components. As we can see, this highly integrated optical sensing microfluidic device opens a new door of sensing, where individual components are integrated into the device itself2.
Electro Based Sensing
Electrochemical sensing is typically based on electrical property modulations of the analyte species that undergo redox reactions. These measurements are then used for the detection of the electroactive species. The working principle of electrochemical sensing is based on an induced electrical current caused from the oxidation or reduction of electroactive species. Detection can be watched in real time via current vs time graphs of the electrode. There are different types of electrochemical methods per applications such as potentiometric detection or amperometry detection 4.
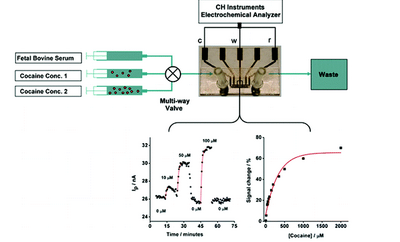
Seen in Figure 6 is an electrochemical sensor design used for real time detection of cocaine in a blood sample. It incorporates three gold working electrodes, a platinum reference electrode and a platinum counter electrode within a detection chamber. The integrated sensor targets specific DNA aptamers that fold and generate an electrochemical signal.3
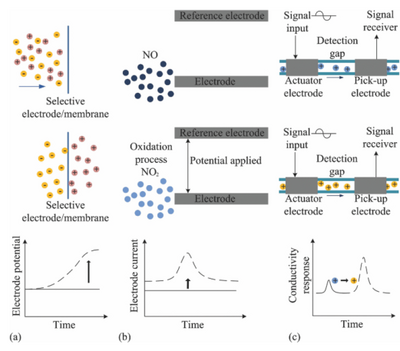
The working methods of electrochemical detection are subdivided into three areas: amperometric, potentiometric, and conductometic. Amperometric sensing is based on an induced electrical current from oxidation or reduction of electroactive species. Sensitive detection can be obtained through cyclic voltammetry, a type of potentiodynamic electrochemical measurement. Potentiometric detection is realized by monitoring the potential of an ion-selective diodes against a reference electrode. As selective ions pass through a membrane, the charge separation causes a potential between the reference and working electrode. Lastly, conductometric detection is used to monitor the conductivity of a zone as it affected by charged species. Different species will bring different conductive responses, and therefore can be used to detection purposes. These detection processes can be summarized below Figure 7, where (a) is amperometric, (b) is potentiometric, and (c) is conductometic 1.
Along with electrochemical based sensing, there has been recent innovation into electrical sensing for microfluidics. Specifically, resistive, conductive,and capacitive sensing have all been integrated into microfluidics with great success. In resistive sensing, a resistor can be used as a flow or temperature sensor. As liquids of varying thermal conductivity flow over a resistor, the temperature of the resistor will fluctuate, resulting in changes to the output current. This has been used to detect changes in liquid concentration flow through a sample7.
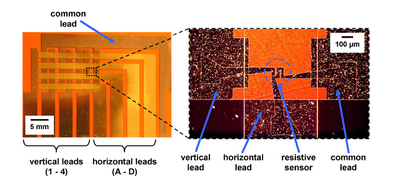
In Figure 8, we see an integrated microfluidic device with a resistive sensor.
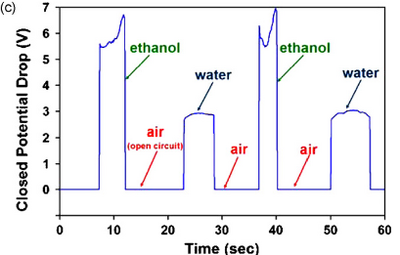
In conductive sensing, constant current is applied between two leads and monitored. When conductive fluid flows through the leads, the system acts like an open circuit. When a conductive liquid fills the gap, the circuit is closed and a potential drop that is proportional to the conductivity of the liquid is detected. Because of this, conductive sensing can be used to easily detect liquids of different conductivity. In Figure 9, conductive sensing was used to distinguish between ethanol and water. We can see the sharp peak and signal differences between the pair. Similarly, in capacitive sensing a constant potential is applied between two electrodes and the current is measured as a function of time. As a fluid with a different dielectric constant passes through, a sharp and sudden current spike is induced, and the signal can be monitored7.
Mass Spectrometry Sensing
Mass spectrometry is being compelled with microfluidic devices for highly selective and sensitive sensing applications. Mass spectrometry is able to detect the paths of ions in electric or magnetic fields and most commonly used for protein separation and identification of protein fragmentation patterns. Mass spectrometry sensing is not currently widely used in microfluidics, but substantial baseline research is being carried out. The coupling with microfluidic systems offers high throughput processing and multiplexing ability 1.
References
1. Jing Wu, Min Gu, "Microfluidic sensing: state of the art fabrication and detection techniques," J. Biomed. Opt. 16(8) 080901 (1 August 2011).https://www.spiedigitallibrary.org/journals/Journal-of-Biomedical-Optics/volume-16/issue-08/080901/Microfluidic-sensing--state-of-the-art-fabrication-and-detection/10.1117/1.3607430.full?SSO=1
2. Kuswandi, B., Nuriman, U., Huskens, J., & Verboom, W. (2007). Optial sensing systems for microfluidic devices: a review. Analytica chimica acta, 601(2), 141-155. https://doi.org/10.1016/j.aca.2007.08.046
3. Continuous, Real-Time Monitoring of Cocaine in Undiluted Blood Serum via a Microfluidic, Electrochemical Aptamer-Based Sensor. James S. Swensen, Yi Xiao, Brian S. Ferguson, Arica A. Lubin, Rebecca Y. Lai, Alan J. Heeger, Kevin W. Plaxco, and H. Tom. Soh.Journal of the American Chemical Society 2009 131 (12), 4262-4266 .DOI: 10.1021/ja806531z. https://pubs.acs.org/doi/pdf/10.1021/ja806531z
4.Rossier J., Reymond F., Michel P. E., “Polymer microfluidic chips for electrochemical and biochemical analyses,” Electrophoresis, 23 858 –867 (2002). https://doi.org/10.1002/1522-2683(200203)23:6<858::AID-ELPS858>3.0.CO;2-3
5.Armani A. M., Kulkarni R. P., Fraser S. E., Flagan R. C., Vahala K. J., “Label-free, single-molecule detection with optical microcavities,” Science, 317 783 –787 (2007). https://doi.org/10.1126/science.1145002
6.https://www.youtube.com/watch?v=3NsM9TWgdVI
7.Matthew C. Cole, Paul J.A. Kenis. Multiplexed electrical sensor arrays in microfluidic networks,Sensors and Actuators B: Chemical,Volume 136, Issue 2,2009,Pages 350-358,ISSN 0925-4005,https://doi.org/10.1016/j.snb.2008.12.010.
8. Hongbin Pu, Wang Xiao, Da-Wen Sun, SERS-microfluidic systems: A potential platform for rapid analysis of food contaminants,Trends in Food Science & Technology,Volume 70, 2017,https://doi.org/10.1016/j.tifs.2017.10.001.
