Microfluidic Sensing- Microfluidic Biosensors- Xiao Fan
Microfluidic biosensors
Biosensor is a kind of analytical device which combines biological sensitive recognition element (such as nucleic acids, enzymes, antibodies) with a physicochemical detector for analytes analysis within an experimental sample.[1] The integration of microfluidic device and biosensor technologies provides new opportunities for biosensing applications such as; portability, real-time detection, increased sensitivity, and selectivity.[2] A lot of microfluidic devices have been developed for biosensor applications. The three major groups of microfluidic biosensors include DNA-based microfluidic biosensors, enzymes-based microfluidic biosensors, and microfluidic immunosensors.
Video: Microfluidic Biosensors, New Frontier of Diagnosis
DNA-based microfluidic biosensors
DNA-based microfluidic biosensors often immobilize complementary sequences of target nucleic acid within the microfluidic device. Next, samples containing targeted nucleic acids are introduced into the microfluidic channels and the detection processes are achieved in the microfluidic device. The recognition process relies on the base pair of hybridization probes and target nucleic acid in the sample. Base pairing processes usually generate physicochemical signals which can be used to analyze the target DNA in the sample.
Generally, detection of DNA involves the amplification of the sample DNA via polymerase chain reaction (PCR) and the subsequent detection of DNA fragments based on their electrophoretic mobility on a gel substrate.[3] By developing microfluidic device, polymerase chain reaction (PCR) and the subsequent detection steps can be integrated into certain microfluidic platforms, reducing risks of sample loss and contamination in general processes.
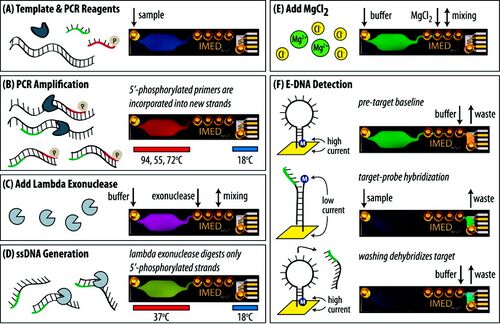
Figure 1 shows an overview of the Integrated Microfluidic Electrochemical DNA (IMED) sensor, which integrates three biochemical functionalities for DNA sensing: symmetric PCR amplification, enzymatic single-stranded DNA generation, and sequence-specific electrochemical detection. The microfluidic device is fabricated using a layer of PDMS and two glass wafers (as shown on the right side in Figure. 1A). In the sensing process, DNA and PCR reagents are introduced into the PCR chamber to rapidly amplify DNA. During PCR, the reverse primers generate phosphorylated strands, which are subsequently and selectively digested by lambda exonuclease to efficiently produce ssDNA targets for detection. MgCl2 is then added for optimal hybridization. Sequence-specific electrochemical detection is performed with electrochemical DNA (E-DNA) probes where target hybridization causes a change in the redox current of the prob. This could be detected via alternating current voltammetry (ACV). The detection limit of this microfluidic device is less than 10 aM when measuring genomic DNA from Salmonella enterica serovar Typhimurium LT2.[4]
Enzyme-based microfluidic biosensors
Enzyme, which is natural occurring proteins that catalyze biologically chemical reactions, are a popular type of bioreceptor used in microfluidic biosensors. Their selection is due to their specific binding capabilities and catalytic activity. In an enzyme-based microfluidic biosensor, enzymes are typically immobilized onto a suitable transducer within the microfluidic device. This setup produces a specific signal such as optical, electrochemical, or colorimetric upon reacting with a target analyte within the microfluidic channels.[5] These signals can be used to measure important health parameters, like cholesterol and blood sugar levels. Monitoring these levels is crucial because they are linked to various diseases.

An example of an enzyme-based microfluidic biosensor is shown in Figure 2. A droplet-based microfluidic biosensor is fabricated based on poly (dimethylsiloxane) (PDMS) using soft lithograph. Glucose oxidase (GOx) is the analyte, which catalyzes the oxidation of β-D-glucose to the product H2O2. In the testing process, the sample stream contains a combination of enzyme GOx and substrate glucose and is subsequently segmented into serial of droplets through intersecting streams of mineral oil. The enzyme activity of GOx can then be evaluated by measuring the concentration of reaction product hydrogen peroxide. Since hydrogen peroxide is electroactive, the enzyme activity of GOx is electrochemically detected based on measuring the electrochemical current on a Pt-black microelectrode responding to various glucose concentrations. This device provides a highly sensitive yet low-cost glucose sensor with linear response up to 43.5 mM.[6]
Microfluidic immunosensors
Microfluidic immunosensors are based on the immunochemical reactions of antigen and antibody. Usually, antibody is spatially in contact with a transducer surface as recognition element to identify the antigen in the sample. Enzyme linked immunosorbent assay (ELISA) is the most widely used method for detection. Additionally, some microfluidic immunosensors can detect immunocomplex formation by changes in current, resistance, refractive index, etc.
Figure. 3 shows a microfluidic immunosensor using microfluidic enzyme linked immunosorbent assay (ELISA) platform for detection of antigen. The device is fabricated by three-layer PDMS/glass and contains four parallel assay units. In the sensing process, the capture antibody (Ab1) was first flowed through the assay chamber where the surface was silanized with APTES to enhance the immobilization of Ab1 through electrostatic interaction between the negatively charged antibodies and the positively charged amine group of 3-aminopropyl triethoxysilane (APTES). The antigen can be captured, recognized by the biotinylated detection antibody (BAb2), and quantified using b-galactosidase (b-gal) as the reporter enzyme for chemi-fluorescence detection.[7]

Figure.4 illustrates another example of immunosensors using antibodies as the biological recognition element for the detection of antigen. The microfluidic chip was fabricated by using three layers of PDMS and a glass substrate. In the testing process, the microfluidic chip is coupled with the SPR phase imaging system. Anti-rabbit IgG solution is constantly pumped through the microfluidic channel for immobilization. Then the rabbit IgG sample was injected into the microchannels. Interaction between rabbit IgG with immobilized anti-rabbit IgG have been successfully measured by using employing SPR phase imaging. The relation between phase difference and the concentrations of rabbit IgG can be obtained. The detection limit could be 1×10-4 mg/ml (0.67 nM).[8]

Reference
1. Nguyen, H. H.; Lee, S. H.; Lee, U. J.; Fermin, C. D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12 (1), 121. https://doi.org/10.3390/ma12010121.
2. Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-On-a-Chip and Sensing Applications. Sensors (Basel, Switzerland) 2015, 15 (12), 30011–30031. https://doi.org/10.3390/s151229783.
3. Choi, S.; Goryll, M.; Yi, L.; Pak Kin Wong; Chae, J. Microfluidic-Based Biosensors toward Point-of-Care Detection of Nucleic Acids and Proteins. 2011, 10 (2), 231–247. https://doi.org/10.1007/s10404-010-0638-8.
4. Ferguson, B. S.; Buchsbaum, S. F.; Swensen, J. S.; Hsieh, K.; Lou, X.; Soh, H. T. Integrated Microfluidic Electrochemical DNA Sensor. Analytical Chemistry 2009, 81 (15), 6503–6508. https://doi.org/10.1021/ac900923e.
5. Kumar, S.; Kumar, S.; Ali, Md. A.; Anand, P.; Agrawal, V. V.; John, R.; Maji, S.; Malhotra, B. D. Microfluidic-Integrated Biosensors: Prospects for Point-of-Care Diagnostics. Biotechnology Journal 2013, 8 (11), 1267–1279. https://doi.org/10.1002/biot.201200386.
6. Gu, S.; Lu, Y.; Ding, Y.; Li, L.; Song, H.; Wang, J.; Wu, Q. A Droplet-Based Microfluidic Electrochemical Sensor Using Platinum-Black Microelectrode and Its Application in High Sensitive Glucose Sensing. Biosensors and Bioelectronics 2014, 55, 106–112. https://doi.org/10.1016/j.bios.2013.12.002.
7. Wang, T.; Zhang, M.; Dreher, D. D.; Zeng, Y. Ultrasensitive Microfluidic Solid-Phase ELISA Using an Actuatable Microwell-Patterned PDMS Chip. Lab on a Chip 2013, 13 (21), 4190. https://doi.org/10.1039/c3lc50783a.
8. Lee, K.-H.; Su, Y.-D.; Chen, S.-J.; Tseng, F.-G.; Lee, G.-B. Microfluidic Systems Integrated with Two-Dimensional Surface Plasmon Resonance Phase Imaging Systems for Microarray Immunoassay. Biosensors and Bioelectronics 2007, 23 (4), 466–472. https://doi.org/10.1016/j.bios.2007.05.007.
