Mice as Animal Models in Tissue Engineering by Jack LaBelle
Background
Mice have been used as animal models in research as far back as the 1500s when English Doctor William Harvey used them to study blood circulation and reproduction [9]. Since then, mice have become the most common animal model used in research and are used in fields including genetics, psychology, and medicine. Due to their similar genetic, biological, and behavioral characteristics with humans, mice have developed a crucial role in drug screenings, and have been used to study a wide range of medical problems including Alzheimer's disease, leukemia, and muscular dystrophy [10].
Motivation in in Tissue Engineering
Why Mice?
Mice exhibit numerous similarities to humans both genetically and biologically, which make them an excellent model in tissue engineering research. The mouse genome has 97% homology with the human genome, making them excellent candidates for genetic studies [2]. Another reason why mice are so commonly used in research is the low cost. The cost of a laboratory mouse typically ranges from $18-66 depending on age and sex, with a generally low cost of maintenance and housing per mouse [14]. With a short lifespan (~2 years) and very high reproductive rates, mice allow researchers to perform experiments on multiple generations of mice in a short time period. Additionally, laboratory mice are mostly inbred, which enables all of the test specimens to be nearly identical genetically.
Types of Mice Used
Nude Mice

Nude mice are one of the most common types of laboratory mice that are used in research. They are a type of genetically mutated mice that have a disrupted FOXN1 gene which results in a missing thymus and a lack of hair. The lack of a thymus means that the nude mice cannot create mature T lymphocytes, and therefore have a greatly inhibited immune system. The reduced immune response is very important for research because it does not allow for any allograft or xenograft rejections in the mice. This allows scientists to to implant human tumors or other tissues into the mice and study their properties without fear of host rejection [3].
Knockout Mice
Knockout mice are another frequently used type of laboratory mice in which a specific gene is made inoperative or "knocked out." This allows researchers to examine the function of certain genes by comparing the mouse with the "knocked out" gene to a mouse that is normally expressing that gene. These mice are very useful in drug screening because they allow researchers to understand how a certain gene affects a drug and its interaction with the disease in mice, and consequently allows inference into how humans will be affected under similar situations [2].
Other Mice
Numerous other strains of mice are used for a wide range of medical purposes. There are transgenic mice in which a foreign genes, such as an oncogene, is inserted into the mice for observation. Similar to the nude and knockout mice, transgenic mice are highly flexible and can be used to study diseases ranging from cancer to Parkinson's. There are also obese mice and non-obese diabetic mice, both of which are mutant strains of mice used to study diabetes. Severely combined immunodeficient (SCID) mice are another form of a mutant strain in which both T and B cells are absent and there is negligible immune response. They are especially useful in transplants as well as testing the safety of vaccines for use in immunocompromised humans [5].
Applications
Skin Engineering
Bio-engineered skin has emerged as an important topic of research in the past two decades in response to burn coverage, chronic ulcers, and various other skin diseases. Immunodeficient mice have served an important role in this research as they are able to accept the human skin grafting without any rejection or negative immune response [4].
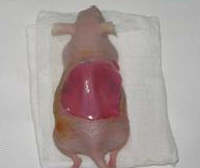
Although many cases of damaged or missing skin can be resolved by using autografts from healthy portions of the patient's skin, this can be difficult in situations where more than 50% of the body surface area are affected. In these situations, such as with severely affected burn victims, bio-engineered skin is an essential alternative to autografts for covering the affected sites. Skin-humanized mice were used by Luis Martínez-Santamaría and his research team in Madrid, Spain, to develop a dermoepidermal equivalent to human skin that is able to artificially reproduce the normal healing process. The mouse model is created by obtaining a skin sample from a wound created on the back of the mouse. The skin is devitalized through various freezing and thawing cycles, and is used to as a protective dressing after the artificial skin has been transplanted. Once the grafting process is complete, the protective skin layer naturally sheds in approximately 2-3 weeks, and the mouse is left with the integrated artificial skin. The skin-humanized mice were extremely important in this study because they were able to confirm the regenerative properties and proper differentiation of the bio-engineered skin because the transplanted skin was still apparent after 4 weeks which is the normal replacement cycle of the epidermis. These skin-humanized mice models are created in a similar way to study other skin diseases such as recessive dystrophic epidermolysis bullosa (RDEB). In cases like this, a skin biopsy would be taken from a patient with RDEB and used to create bioengineered RDEB skin which is then transplanted into the mouse for observation [4].
Drug Screening
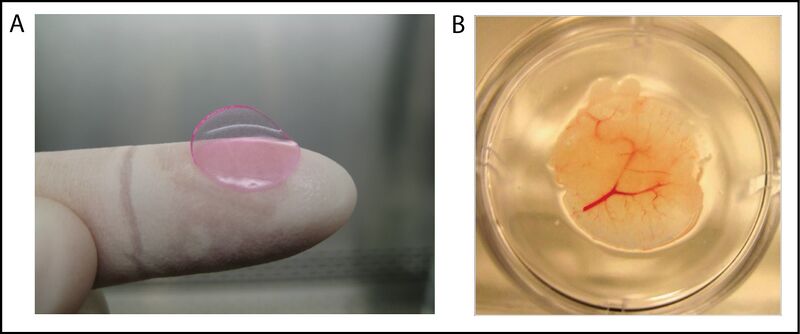
New drugs have to undergo a relentless process of getting approved by the FDA, and take approximately 12 years to get from the invention stage to being sold on the market. The preclinical trials involve numerous animal testing to show the biological activity of the drug and confirm the safety of its usage in humans. Since no animals process drugs identically to humans, it is difficult to be certain exactly how new drugs will act in clinical trials [2]. For this reason, tissue engineered human livers have been implanted into mice to get a more accurate drug screening process. This is a much safer method as it can avoid harmful metabolites from being processed in human testing that may not have been observed in the drug breakdown of the animal liver. These tissue engineered livers that have been developed by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) are referred to as HEALs (human ectopic artificial livers). These livers are created by growing human hepatocytes with mouse fibroblasts in vitro for one week, followed by mixing with human endothelial cells, and lastly encapsulating the cell combination in PEG-DA (polyethylene glycol diacrylate) scaffolds. These HEAL livers can survive without any immune response from the mice, and are able to be implanted into the mice without the removal of their preexisting liver. The HEAL-humanized mice have also shown human specific drug breakdown products that are not apparent in normal mice, which shows that the implanted liver exhibits the functionality of a human liver and can continue to be used to enhance the drug screening process [11].
Neurological Disorders
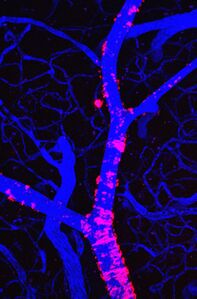
Mice have played an important role in studying degenerative neurological disorders including Parkinson's disease, Huntington's disease, and Alzheimer's disease. Alzheimer's disease in particular has been a major focus of research since it is serious neurodegenerative disease that affects approximately 10% of people aged 65 or older. Alzheimer's disease results in amyloid plaques and neurofibrillary tangles in the brain, which are pathological lesions that are toxic to the neurons in the brain [1]. Although most Alzheimer's patients do not have an observed heritable genetic component, a small percentage of patients do exhibit heritable forms of the disease which make transgenic mouse models ideal candidates for study. The various inherited mutant genes and genetic traits have been observed in different variations of transgenic mice and allow researchers to investigate the disease pathology [2]. Another approach that has been used to gain insight on the development and progression of Alzheimer's disease as well as other neurological disorders is multiphoton microscopy (MPH) on transgenic mouse models. This approach involves a implanting a cranial window into the skull of the mouse model so that there can be optical access to the blood vessels, neurons, and plaques for the MPH [6]. Fluorescent injections (e.g. methoxy-XO4) are also used to easily identify proteins and clots such as the amyloid-beta peptide which is the main protein found in amyloid plaques. Although the mouse models a lot information on disease pathology and specific gene affects on neurological disorders including Alzheimer's disease, individual mouse models are unable to reproduce the full phenotype of the disease and therefore require numerous models to obtain a comprehensive understanding of the disease [10].
Cancer Research
Due to their immunodeficient characteristics, certain strains of laboratory mice provide an ideal animal model for cancer research. Immunocompromised mice such as SCIDs and nude mice are able to accept human tumor cell xenografts and allow the study of malignant transformation, metastasis, and therapeutic response [3]. Genetically engineered mice (GEM), which include knockout mice and transgenic mice, are also used heavily in cancer research. In these mice, genes related to malignancy are either altered, deleted, or overexpressed, and provide an alternative approach to cancer research [5].
The immunocompromised mice with implanted tumor cells provide more useful information on therapeutic drug response since actual human tissue is being used in these mice. This allows actual human response to be examined before and after drug therapy, with the capability of testing multiple therapies on a singular xenograft. Xenografts using immunodeficient mice have shown significant results especially in multiple myeloma research. Mice trials showed positive response to the proteasome inhibitor, bortezomib, and have been successful in clinical trials. Xenograft research has led to successful clinincal trials of numerous other drugs including herceptin and bevacizumab which are used for breast cancer and renal carcinoma, respectively. Setbacks of the xenograft method in immunodeficient mice are that the mice do not have T-cells and the lymphocyte-mediated response cannot be observed, and they are very time consuming to monitor and can take several months for the transplanted tumor to develop. As a compliment to xenografting, GEM models provide a more useful approach to understanding the role of specific genes in tumor development over time. They allow the examination of different therapeutic methods at various stages in the tumor development, and allow for the reproduction of genetic abnormalities found in human tumors. Disadvantages of GEM models in cancer research are that the complexity human tumors cannot be reliably copied, and mouse tumors will not necessarily predict the same response that will occur in a human tumor [3].
Challenges
Although mice provide a an excellent animal model that can be used in multiple fields of scientific research, there are still challenges associated with their use. Creating genetically modified mice for various neurological or other medical applications can be very complex and not always produce the desired results. For example, there have been numerous cases of knockout or transgenic mice that are modified to genetically mimic a certain disease but do not express the features of that disease [12]. Also, although human implanted tissues have been transplanted into mice for research in some circumstances, this is not always the case. When drug screenings or others medical testing are performed on mice, it does not always accurately represent the response that will be found in humans [3].
History
1500s - Mice were used by William Harvey to study reproduction and blood circulation [9].
1907 - Harvard undergraduate, Clarence T. Little, discovered that the inheritance of mouse coat color did not follow Mendelian rules [14].
1962 - Nude mice were first discovered by Dr. N. R. Grist at Ruchill Hospital's Brownlee virology laboratory in Glasgow [7].
1970s - Experiments were performed by W. T. Green, M.D. to generate new cartilage by implanting spicules of bone into nude mice that were seeded with chondrocytes [7].
1974 - The first genetically modified animal was created by Rudolf Jaenisch by inserting a DNA virus into an early-stage mouse embryo and showing that the inserted genes were present in every cell [9].
1980s - A method of altering or removing any single gene from the mouse genome was developed by Mario R. Capecchi, Ph.D. [8].
1981 - Purified DNA was injected into a single-cell mouse embryo by Frank Ruddle of Yale. This resulted in transmission of the genetic material to subsequent generations for the first time [7].
1989 - The first recorded knockout mouse was created by Mario R. Capecchi, Martin Evans, and Oliver Smithies in 1989, for which they were awarded the 2007 Nobel Prize in Physiology or Medicine [9].
1997 - The "Vacanti mouse" was created by Charles Vacanti at the University of Massachusetts Medical Center. The "Vacanti mouse" is known for having human ear shaped cartilage attached to its back [8].
Sources
[1] Willerth, Stephanie M. "Neural Tissue Engineering Using Embryonic And Induced Pluripotent Stem Cells". Stem Cell Research & Therapy 2.2 (2011): 17. doi: 10.1186/scrt58.
[2] Harper, Alex. "Mouse Models Of Neurological Disorders—A Comparison Of Heritable And Acquired Traits". Biochimica et Biophysica Acta (2010). doi: 10.1016/j.bbadis.2010.05.009.
[3] Richmond, A., and Y. Su. "Mouse Xenograft Models Vs GEM Models For Human Cancer Therapeutics". Disease Models and Mechanisms 1.2-3 (2008): 78-82. doi: 10.1242/dmm.000976.
[4] Martínez-Santamaría L, et al. "Skin Bioengineering: Preclinical And Clinical Applications". Actas Dermosifiliograficas 103.1 (2012): 5-11. doi: 10.1016/j.adengl.2011.03.016
[5] Greenberg, N., et al. "Prostate Cancer In A Transgenic Mouse". Proceeding of the National Academy of Sciences 92 (1995): 3439-3443.
[6] Bacskai, B. J. et al. "Four-Dimensional Multiphoton Imaging Of Brain Entry, Amyloid Binding, And Clearance Of An Amyloid- Ligand In Transgenic Mice". Proceedings of the National Academy of Sciences 100.21 (2003): 12462-12467. doi: 10.1073/pnas.2034101100
[7] Vacanti, C. "The History Of Tissue Engineering". Journal of Cellular and Molecular Medicing 10.3 (2007): 569-576.
[8] Meyer, Ulrich. "The History Of Tissue Engineering And Regenerative Medicine In Perspective". Fundamentals of Tissue Engineering and Regenerative Medicine (2009): 5-12. doi: 10.1007/978-3-540-77755-7_1
[9] Hedrich, Hans. "The House Mouse As A Laboratory Model: A Historical Perspective". The Laboratory Mouse. Elsevier Science. ISBN 9780080542539.
[10] "Of Mice And Microscopy - Promising Insights For Alzheimer’S Research". National Institute of Biomedical Imaging and Bioengineering. N.p., 2007.
[11] "A Tale of Two Livers - Developing an Innovative Drug Screening Tool". National Institute of Biomedical Imaging and Bioengineering. N.p., 2012.
[12] Hendricks, M. "The Mouse Model: Less than Perfect, Still Invaluable". Johns Hopkins Medicine: Institute for Basic Biomedical Sciences. N.p., 2010.
[13] "How and Why Mouse Cancer Models are Used". National Cancer Institute: U.S. National Institutes of Health. N.p., 2015.
[14] The Jackson Laboratory. (undated). C57BL/6J. Retrieved from http://jaxmice.jax.org/strain/000664.html
