Targeted approaches
Dissecting the mosquito immune pathways. Pioneering studies in the fly Drosophila melanogaster contributed to the detailed understanding of innate immunity and showed that the underlying mechanisms have largely been conserved through the course of metazoan evolution [4]. These studies revealed most of the components of two conserved immune signaling pathways, Toll and Imd, that are utilized by the fly to respond to bacterial and fungal infections (Figure 2A). The availability of the A. gambiae genome sequence [5] previously allowed us to perform a comparative genomic analysis of putative immunity genes between Anopheles and Drosophila [6]. Although the majority of the intracellular components of the Toll and Imd pathways are conserved between the two organisms (Figure 2B), a number of differences – which may have significant impact on immunity mechanisms – were also identified. The most important of these is the absence of the NF-kB-like transcription factor Dif in Anopheles. This suggested that in a functional Toll pathway either REL1 (the ortholog of Dorsal) or REL2 (the ortholog of Relish) might substitute for Dif. We investigated the role of REL2 and other molecules that are possibly implicated in the same signalling pathway in the mosquito immune responses. REL2 regulates the inducible expression of the various antimicrobial peptide genes including CEC1 and the key parasite antagonist, LRIM1 [7]. We showed that the REL2 gene is alternatively spliced, resulting in two protein isoforms that are differentially implicated in defense against Gram-positive or Gram-negative bacteria. Thus, through alternative splicing Anopheles uses a single gene to mediate reactions for which Drosophila employs two genes, Relish and Dif. The REL2 pathway is also involved in the control of Plasmodium parasite infection of the mosquito midgut. Silencing of the pathway drastically increases the parasite numbers that successfully develop into oocysts.
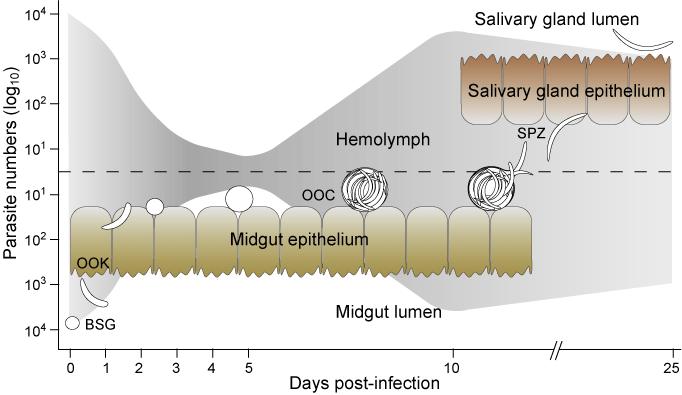
Deciphering the molecular mechanisms affecting ookinete survival in the mosquito. Vector immune responses are responsible, at least in part, for the major parasite losses during parasite development. We now know, largely from functional studies in our laboratory, that even what is called “susceptible” A. gambiae mosquitoes effectively kill a large number of invading ookinetes (80 %), which are most probably cleared by lysis. Two genes, LRIM1 and TEP1, were shown to be strongly involved in ookinete killing in susceptible mosquitoes (G3 strain). LRIM1 expression was strongly induced in mosquito midguts and carcasses in response to the invasion of the mosquito midgut epithelium by Plasmodium ookinetes. The transient KD of LRIM1 in susceptible mosquitoes by RNAi resulted in approximately a four-fold increase in the number of parasites that successfully develop in the mosquito midguts, suggesting that LRIM1 is involved in parasite killing. TEP1 is as a bona fide pattern-recognition receptor that binds to the surface of bacteria and Plasmodium ookinetes. In a collaborative work led by E. Levashina (former staff scientist in our lab and currently an independent scientist at Institut de Biologie Moléculaire et Cellulaire, CNRS in Strasbourg) in vivo RNAi was used to KD TEP1 in adult susceptible mosquitoes and we demonstrated that this binding promotes two distinct immune reactions: phagocytosis of bacteria [8] and killing of Plasmodium ookinetes in the mosquito midgut [9]. These reactions are reminiscent of functions of complement factors in vertebrates [10]. To complete the functional analysis of TEP1 in vivo, we have established TEP1 gain-of-function transgenic mosquitoes, which overexpress the TEP1 gene under the control of the ubiquitous D. melanogaster heat shock protein 70 promoter. Two transgenic lines were obtained. Surprisingly, in one of the two lines, expression of the endogenous TEP1 and of the transgene was lost in a heat-shock independent manner. The knockout of TEP1 by transgenesis gave the same phenotype as that observed in mosquitoes in which TEP1 was transiently knocked down by RNAi. We are currently studying possible mechanisms of this inactivation. Importantly, overexpression of TEP1 in the second transgenic line lead to a significant decrease in parasite numbers suggesting that TEP1 is not only necessary but also sufficient to promote killing of parasites. Functional studies of this type will be facilitated in the future by conditional transgenic protocols of the type developed recently in a collaboration involving former members of the lab [11].
In extreme cases, genetic selection of formerly susceptible mosquitoes can give rise to complete refractoriness: An L3-5 strain of A. gambiae melanizes all ookinetes in the basal labyrinth of the midgut epithelium, while another refractory strain lyses the ookinetes in the cytosol of the midgut epithelial cells. Studies from our own and other laboratories, employing mapping of quantitative trait loci (QTL) that affect the L3-5 phenotype, revealed that different QTLs are linked to melanization of two different but related species of Plasmodium, P. cynomolgi B and P. cynomolgi Ceylon. This suggests that different genetic loci may be involved in L3-5 responses to different malaria parasites [12, 13]. We are currently developing fluorescence polarization SNP genotyping methods for fine scale QTL mapping to further pinpoint the genetic basis of the melanization phenotype in the L3-5 strain.
In general, melanization in insects requires the limited proteolytic cleavage of prophenoloxidase (PPO) into active phenoloxidase (PO) by PPO-activating enzymes (PPAEs), a reaction believed to be influenced, both positively and negatively by additional factors including serine protease homologs (SPHs), serpins and C-type lectins (CTL, see below). The identification and biochemical study of components of the PPO-activating cascade is an important goal for understanding how melanization is regulated in A. gambiae. Nine PPO genes have been identified in the A. gambiae genome, in contrast to merely 3 PPO genes in D. melanogaster. These 9 PPO genes are transcribed at different developmental stages, showing partly overlapping expression profiles. Expression of several PPOs is also induced during blood meal digestion. We are currently analysing the function of PPO genes in the melanization process using in vivo RNAi. Our first results indicate that a combination of several PPO is involved in parasite melanization. PPOs are activated by PPAEs, which are trypsin-like serine proteases containing an amino-terminal CLIP-domain with an unknown function. Of the 41 CLIP-domain encoding genes identified in A. gambiae, subfamily CLIPB, containing 17 genes, is of special importance due to significant structural identity with other insect PPAEs involved in melanization. Functional studies using RNAi identified seven CLIPBs involved in ookinete melanization and two in ookinete killing through a distinct mechanism, probably lysis. These results suggest the involvement of a serine protease cascade in parasite killing. The putative interactions of these CLIPBs with each other and/or with other identified immune proteins are under investigation in order to understand how the cascade regulates downstream effector mechanisms.
A group of proteins that may interact with CLIPBs in the melanization response are a group of serine-protease inhibitors, called serpins. Over the last few years several serpins have been shown to negatively regulate the PPO cascade, by directly inhibiting PPAE. One of these serpins is Spn27A from D. melanogaster [14, 15] which forms an orthologous group with three A. gambiae genes (SPRN1, 2 and 3). Reverse genetic analysis revealed that SRPN2 is the functional Spn27A homolog in adult female mosquitoes, causing spontaneous melanization in the mosquito. Depletion of SRPN2 from the hemolymph also negatively affects vector competence towards P. berghei infection, by strongly reducing the number of developing oocysts. Interestingly, the mosquito SRPN2 gene cannot rescue the melanization phenotype of the Spn27A mutant fly strain, suggesting differences in the downstream targets that these serpins inhibit.
On the other hand, previous studies from several insect species revealed that melanization is positively regulated by members of the CTL and SPH subfamilies. The picture seems to be reversed in A. gambiae, in which functional analysis of the CTL genes using RNAi allowed the identification of two CTLs, CTL4 and CTLMA2 that block parasite melanization, consequently protecting Plasmodium ookinetes from this potent immune response [7]. The mechanism(s), by which these CTLs block melanization, is not yet elucidated. However, by analogy to published data, we hypothesize that CTL4 and CTLMA2 might associate with SPH of the CLIPA subfamily to form functional complexes which inhibit the PPO cascade. To this purpose, we functionally analysed eight out of ten CLIPA genes using RNAi in adult susceptible mosquitoes and scored the effect of the different gene KD on Plasmodium survival, with the aim of identifying CLIPA genes whose knockdown phenotype is similar to that previously described for CTL4 and CTLMA2. The preliminary results were intriguing and revealed that different CLIPA proteins have opposing functions in Plasmodium melanization. Work is in progress to investigate the potential interactions between CTL4, CTLMA2 and the CLIPA proteins that inhibit melanization. The aim is to decipher the role of these lectins in melanization and to shed light on the mechanisms that govern, at least in part, successful parasite survival in its vector.
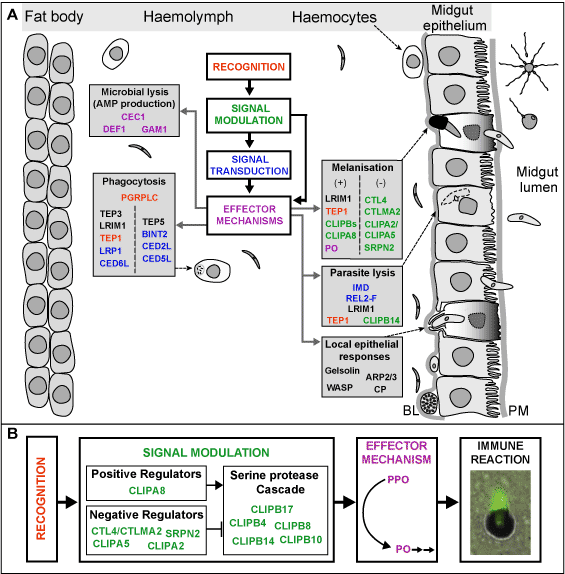
Figure 2
- Hoffmann JA and Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002 Feb;3(2):121-6. DOI:10.1038/ni0202-121 | PubMed ID:11812988 | HubMed [hoffmann2002]
-
pmid= 12364791 [holt2002]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Müller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, and Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002 Oct 4;298(5591):159-65. DOI:10.1126/science.1077136 | PubMed ID:12364793 | HubMed [christophides2002]
- Osta MA, Christophides GK, and Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004 Mar 26;303(5666):2030-2. DOI:10.1126/science.1091789 | PubMed ID:15044804 | HubMed [osta2004]
- Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, and Kafatos FC. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001 Mar 9;104(5):709-18. DOI:10.1016/s0092-8674(01)00267-7 | PubMed ID:11257225 | HubMed [levashina2001]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, and Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004 Mar 5;116(5):661-70. DOI:10.1016/s0092-8674(04)00173-4 | PubMed ID:15006349 | HubMed [blandin2004]
- Blandin S and Levashina EA. Thioester-containing proteins and insect immunity. Mol Immunol. 2004 Feb;40(12):903-8. DOI:10.1016/j.molimm.2003.10.010 | PubMed ID:14698229 | HubMed [blandin2004a]
- Lycett GJ, Kafatos FC, and Loukeris TG. Conditional expression in the malaria mosquito Anopheles stephensi with Tet-On and Tet-Off systems. Genetics. 2004 Aug;167(4):1781-90. DOI:10.1534/genetics.104.028175 | PubMed ID:15342516 | HubMed [lycett2004]
- Zheng L, Cornel AJ, Wang R, Erfle H, Voss H, Ansorge W, Kafatos FC, and Collins FH. Quantitative trait loci for refractoriness of Anopheles gambiae to Plasmodium cynomolgi B. Science. 1997 Apr 18;276(5311):425-8. DOI:10.1126/science.276.5311.425 | PubMed ID:9103203 | HubMed [zheng1997]
- Zheng L, Wang S, Romans P, Zhao H, Luna C, and Benedict MQ. Quantitative trait loci in Anopheles gambiae controlling the encapsulation response against Plasmodium cynomolgi Ceylon. BMC Genet. 2003 Oct 24;4:16. DOI:10.1186/1471-2156-4-16 | PubMed ID:14577840 | HubMed [zheng2003]
- De Gregorio E, Han SJ, Lee WJ, Baek MJ, Osaki T, Kawabata S, Lee BL, Iwanaga S, Lemaitre B, and Brey PT. An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev Cell. 2002 Oct;3(4):581-92. DOI:10.1016/s1534-5807(02)00267-8 | PubMed ID:12408809 | HubMed [degregorio2002]
- Ligoxygakis P, Pelte N, Ji C, Leclerc V, Duvic B, Belvin M, Jiang H, Hoffmann JA, and Reichhart JM. A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J. 2002 Dec 2;21(23):6330-7. DOI:10.1093/emboj/cdf661 | PubMed ID:12456640 | HubMed [ligoxygakis2002]
All Medline abstracts: PubMed | HubMed
|