Jessica Karen Wong/Notebook/2007-7-5
From OpenWetWare
Jump to navigationJump to search
To Do
- PCR clean and then Digest T9002 with Nsi1
- Run gel of I2056
- Drop off sequencing
T9002
- Heat Shocked Mfe1 digest
- PCR cleaned, DNA concentration was only 19 ng/ul
- Continued the sequential digest
- Used all the Mfe1 digest product (30ul), 12.5 ul water, buffer 3
- Also trying a double digest of Mfe1 and Nsi1 in buffer 2
- 11ul scarred DNA, 31.5 ul water
- PCR cleaned both
- Sequential Digest DNA concentration 10.9 ng/ul
- Double Digest DNA concentration 19 ng/ul
I2056
- Ran gel of the overnight colony PCR
- Have a faint band of the right size
- Overnighted 2 cultures
- One for glycerol and sequencing, one to miniprep for scarring
- Used 5ul Amp and 10ul Tet per 5ml culture
E0240
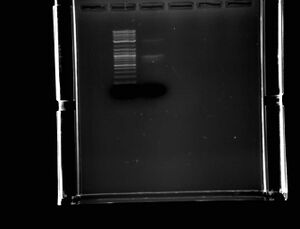
- Did a 100ul preparatory PCR at 53.5
- Fwd primer BB_E0240_F was 38.2nmole added 956ul water
- Rev primer BB_backbone was 38.09nmole added 952ul water
- Ran a gel of the PCR, sample to the right of 2 log ladder
- Faint band of correct size (3kb) but much brighter band that was too small (1kb)
- Didn't use a long enough elongation time
- Will retry with longer elongation time and both vent and phusion polymerases