Jello Microfluidics - Duong Nguyen
Introduction
Jell-O is a desert made of gelatin, a macromolecular protein that can be found in mammals. Gelatin is a derivative of collagen, produced by breaking down the bones and skins of animals. Gelatin is inexpensive, easy to produce and can be stored for a long time without degradation, which makes it a good, simple and easily accessible material for producing low cost microfluidic devices.[1]
Background and Motivation

Many types of materials can be used to fabricate microfluidic devices. PDMS is the most used polymer due to its low cost, transparency, great flexibility and durability. However, it is difficult to fabricate PDMS microfluidic devices outside of a laboratory [2]. It also has been shown that PDMS has no bioactivity and only acts as a holder when the cells are cultured in microdevices [3]. Gelatin is derived from collagen, a common extracellular matrix protein that can be found in bones and connective tissues [4]. Enzymatically crosslinked gelatin has been shown to be biocompatible to human and bacterial cells, which makes it possible to create three dimensional matrices with good cell survival [4]. Gelatin powder is cheap, readily available easy to process. Gelatin microfluidic devices and their mold materials are nontoxic and easy to dispose. By utilizing gelatin, simple, non-toxic and inexpensive microfluidic devices can be obtained in short amount of time and easily used as teaching tools, or in simple microfluidic experiments to demonstrate the characteristics of fluid at microscale level [2].
Methods
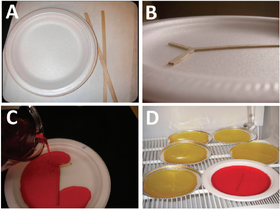
Simple Jell-O Chips
A simple gelatin microfluidic device can be made from Jell-O powder using foam plates, wooden stirrers and double sided tape as mold materials:
Jell-O powder is dissolved in water by heating up the solution. Wooden stirrers are attached to the foam plate using double sided tape to create the patterns of the device. Cooking spray can be used to coat the inside rim of the plate to make it easier to peel the jello after curing. The gelatin solution is poured onto the mold and allowed to solidify at 4°C. After curing, the Jell-O devices can be peeled off and placed on aluminum pans. Inlet and outlet holes can be punctured using a drinking straw to inject the fluid into the channels [2]. These Jell-O chips can be used as simple, fast and inexpensive teaching tools to demonstrate the microfluidics principles in education [2].
Enzymatically Crosslinked Gelatin Microfluidic Devices for Cell Culture
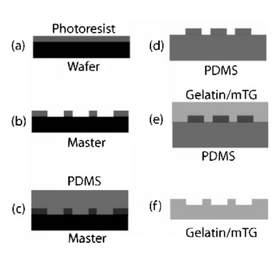
Enzymatically crosslinked gelatin microfluidic devices for cell culture can be created using a poly(dimethyl siloxane) (PDMS) mold. Cells can be cultured in these devices to study the effects of soluble factors and extracellular matrix on cellular morphology [2]. To prepare the mold, a negative photoresist can be spin coated onto the silicon wafers and photomasks is used to produce the patterns on PDMS mold. PDMS can be cured on the master and removed to make a mold for gelatin solution [3]. Gelatin is dissolved in Dulbecco's Phosphate Buffered Saline (DPBS) by heating and 0.02% chloroform is added to the mixture for sterilization. A crosslinking agent, for example, microbial transglutaminase (mTG) can be added to the solution to crosslink gelatin. The resulting mixture is poured over the PDMS molds and allowed to incubate at 37°C for 5 hours [3]. The gels can be removed after cooling to 4°C and heat treated in DPBS at 65°C for 30 minutes to inactivate the enzyme and dialyze chloroform from the gels. A thin PDMS membrane can be placed over the top of the crosslinked gel microchannels to complete the device [3].
Before growing cells, the molded gelatin gels need to be soaked overnight in Dulbecco's Modified Eagle's Medium (DMEM) to prevent the media components from leaching into the gels during culture [3].
Applications
Cell Culture
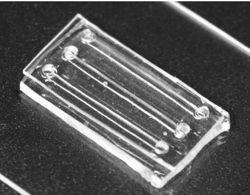
Gelatin has been widely used to modify materials that are inert to cells [5]. Even though gelatin forms thermoreversible gels, it is usually crosslinked with enzymes or chemicals to guarantee its use in biomedicine and improve its strength and durability[5]. However, chemically crosslinked gelatin gels usually have adverse effects on cells [5]. Enzymatically crosslinked gelatin gels have been shown to be biocompatible to both human and bacterial cells [4]. A fabricating method was developed by Paguirigan and Beebe using gelatin crosslinked with enzyme transglutaminase to make microfluidic devices for culturing cells [2-4]. This device was used to study the morphology of cells under the effects of soluble factors and extracellular matrix [2]. Transglutaminase crosslinked gelatin microdevices can mold photodefinable geometries into a natural biopolymer with no synthetic components [3]. Microfluidic devices made of gelatin crosslinked with transglutaminase can create channels of a few 100 μm to millimeter using soft lithography [6]. When the cells are seeded into these devices, it has been shown that they can grow in the matrix and form 3D structures, while still maintaining fluidic access [3].
Droplet-based Microfluidics

Gelatin is a hydrogel, which can give the microfluidic device hydrophilic surfaces that allow it to fabricate uniform microparticles [6]. Using riboflavin as the crosslinking agent to improve the mechanical and thermal stability of gelatin, it is possible to create a stable microfluidic device with feature size smaller than 100 µm to synthesize oil-in-water droplets and oil-in-oil-in-water double emulsions [6]. The droplets can be varied in size and shape by adjusting the flow rate ratios of the water-to-oil phases. [6] Oil-in-water microfluidic droplets can be used as templates to engineer monodisperse organophilic microparticles, while oil-in-oil-in-water double emulsions can be used as templates, monodisperse hollow or asymetric particles to release organophilic reagents in hydrophobic drug delivery systems [6].
References
[1] Saddler,J.,M., Horsey, P., J.,(1987). The new generation gelatins A review of their history, manufacture and properties, Anaesthesia, Volume 42, pages 998-1004
[2] Yang, C., W., T., Ouellet, E., & Lagally, E. T. (2010). Using Inexpensive Jell-O Chips for Hands-On Microfluidics Education, Analytical Chemistry Feature, 82, 13, 5408-5414
[3] Paguirigan, A., L.,Beebe, D.,J.,(2005). Gelatin based microfluidic devices for cell culture, Lab on a Chip, 6, 407–413
[4] Paguirigan, A., L.,Beebe, D.,J.,(2007). Protocol for the fabrication of enzymatically crosslinked gelatin microchannels for microfluidic cell culture, Nature Protocols, Vol.2, NO.7
[5] Pitingol, G., Riaud, A., Nastruzzi, C., Taly, V., (2019). Gelatin-Coated Microfluidic Channels for 3D Microtissue Formation: On-Chip Production and Characterization, Micromachines, 10, 265
[6] Ma, S.,(2018). Gelatin-based microfluidics device with the feature sizes, Microfluidics and Nanofluidics, 23:35
