Induced Pluripotent Stem Cells and Tissue Engineering, by Emma Durkee
Introduction
Induced pluripotent stem cells (iPSCs) are pluripotent stem cells derived from reprogrammed adult somatic cells. These iPSCs function almost equivalently to embryonic stem cells (ESCs) and can differentiate in vitro into each of the three germ layers: ectoderm, mesoderm, and endoderm [1]. iPSCs have been utilized in the field of tissue engineering because they have many valuable characteristics, such as the ability to control their differentiation and reproduction into any desired type of human cell and their self-renewing capabilities [1]. Therefore, scientists can take cells from someone’s blood, skin, and more, reprogram them to become iPSCs, and then use the iPSCs to produce the cell type of a desired damaged tissue [7].
History

2006
The discovery of iPSCs is given credit to the scientists Kazutoshi Takahashi and Shinya Yamanaka of Japan who successfully transformed mouse adult fibroblasts into cells that expressed the embryonic stem cell markers and developed an embryonic-like structure and quality in 2006 [15]. These cells were termed induced pluripotent stem cells. Takahashi and Yamanaka chose and then evaluated the connection of 24 genes to pluripotency. They carried this out by delivering these genes into the source cells through retroviral transduction. The presence of all 24 genes gave rise to iPSCs. Takahashi and Yamanaka then expressed different combinations of these 24 genes in order to eliminate any genes that were not necessary to achieve pluripotency. It was found that the Oct4, Sox2, Klf4 and c-Myc genes were all necessary to produce iPSCs, whereas the other 20 genes were not [15]. Although iPSCs were formed, the process used to generate iPSCs was inefficient due to a low yield of product cells. Takahashi and Yamanaka hypothesized that when the amount of genes delivered into the source cells increased the amount of iPSCs would increase, but their ability to reproduce would be lost [15].
2007
Improving upon his original work, Yamanaka found that by replacing the Fbxo15 gene with either Nanog or Oct4, the iPSCs generated were more comparable to embryonic stem cells molecularly and functionally [16]. Also in 2007 iPSCs were formed from the first human cells by multiple lab groups. A lab group led by Takahashi successfully created iPSCs from human dermal fibroblasts by delivering the Oct3/4, Sox2, Klf4, and c-Myc genes by retrovirus transfection [16].
2012
In 2012 Shinya Yamanaka, as seen in Figure 2., won half of the Nobel Prize in Physiology or Medicine because of his great contributions to the world of science.
Preparation
Creating iPSCs in vitro utilizing nuclear reprogramming involves the introduction of genes that are vital to the production of transcription factor proteins that regulate embryonic development. This regulation transpires through the activation or deactivation of other genes. Because the four genes inserted by Yamanaka’s lab, Oct4, Sox2, Klf4, and c-Myc, are not naturally expressed in the cells, it is necessary to artificially express them [12]. These reprogramming factors or, sets of genes associated with pluripotency, are inserted into a given source of adult somatic cells, commonly obtained from one's blood or skin cells. The source cells are isolated, cultured, and the reprogramming factors are then introduced by one of the two types of delivery methods as depicted in Figure 3. [1]:
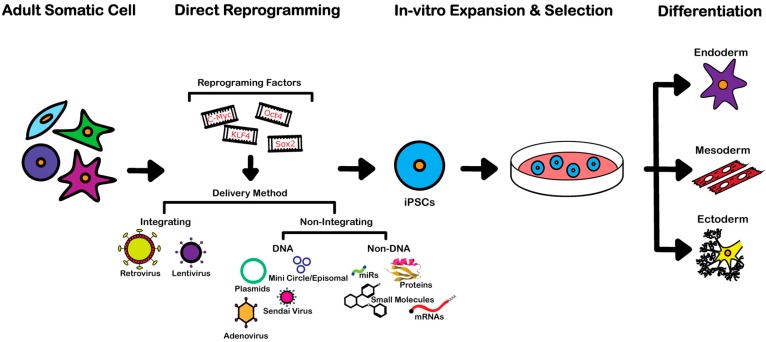
Integration Methods
During integration, the desired genes are inserted into the genome of the host cell. Lentivirus and retrovirus vectors are utilized because they are able to form iPSCs in an almost unlimited amount. On the other hand, this method is risky due to its vulnerability to tumorigenesis and mutagenesis [1]. Tumorigenesis and mutagenesis may occur if viral transgenes are integrated or if the differentiation potential is changed resulting from the vector. Also, c-Myc is a proto-oncogene and its reactivation can cause cancer [1]. This method also has a very low efficiency of reprogramming of under 1% for each vector [1].
Non-Integrating Methods
Non-integrating delivery methods have been used to overcome the genetic abnormalities that have shown to arise when using integrating delivery methods [1].
DNA - Based
- DNA-based non-integrating methods include
- Minicircle Vectors - Minicircle vectors have been used because they are compact and do not consist of any bacterial DNA. This method is also requires only one vector, does not require oncogenes to be utilized, and is FDA approved [1]. Minicircle vectors containing Lin28, Green Fluorescent Protein, Nanog, Sox2, and Oct4 have been delivered into human adipose cells to create iPSCs with a 0.005% yield. There is more work that must be done with this type of vector to truly understand its role in the reprogramming of cells [17].
- Episomal Vectors - Episomal vectors have also been used to successfully generate iPSCs from cord blood cells and peripheral blood cells with an efficiency of 0.005% [17]. They are attractive because they can be manipulated easily [17].
- Sendai viruses - The sendai virus is an RNA virus that does not change the genome of the host cell. This specific virus is used to generate human iPSCs because it has an efficiency of about 1%, which is much higher than other methods, and any iPSCs that use up the genome of the virus from the cytoplasm are easily obtained [1].
- Adenoviruses - Adenoviruses are used for a brief yet high level of expression of the desired genes. Studies have shown that these viruses have an efficiency of about 0.0001% to 0.001% which is much less than the efficiency of viruses that allow for the integration of DNA. It is hypothesized that the efficiency of adenoviruses is so low because the quick expression of genes is not enough to maintain pluripotency [1].
Non - DNA Based
- The non-DNA based non-integrating methods include
- Proteins - Genetically recombined proteins that can enter the membrane of adult somatic cells have been a large area of research when it comes to iPSCs because it would be a cheaper, easier, broader, and faster approach than using a genetically-based approach [1]. On the other hand, using proteins to induce pluripotency requires difficult synthesis and purification.
- Micro RNAs - Introducing microRNAs by transfection, especially mir-200c, mir-302s and mir-369s family microRNAs, has successfully produced iPSCs from both human and mouse somatic cells. This method is promising because it does not require a viral vector [17].
- Small molecules - Small molecules have been successfully utilized to generate iPSCs from somatic mouse cells by affecting the activation or deactivation of certain signaling pathways within the cell. They are advantageous because they are permeable to cells, easy to make, cheap, and often have reversible effects [2]. On the other hand, this method is inconsistent and has a low efficiency [1].
- mRNAs - Altered mRNAs have successfully been used in place of DNA as a reprogramming factor to induce pluripotency in human fibroblasts with an efficiency of about 2% and a decreased anti-viral response [1]. This method is extremely time-consuming and takes much work [17].
Areas of Application
Cardiovascular Disease
Cardiovascular disease is widely encountered throughout the world and includes many conditions including heat arrhythmias, coronary artery disease, congenital heart defects, blood vessel diseases, and more. These disorders are all prime examples of the application of iPSCs due to the limited regenerative ability of adult hearts and lack of donor hearts [8]. It has been shown that human iPSCs have the potential to develop into cardiomyocytes which could then be used to create cardiac patches, heart valves, and blood vessels and restore cardiac function [11]. The most common procedure used to produce cardiomyocytes involves subjecting iPSCs to TGF-β and Wnts differentiation signals during culturing [9].
Neurodegenerative Diseases
Brain diseases are challenging to study because human brains are inaccessible, the brain models for most species are not finished, and there are many difficulties associated with examining post-mortem brain tissue [5]. Many neurodegenerative diseases, such as Huntington’s disease, Alzheimer’s disease, and Parkinson’s disease, have been very attractive for tissue regeneration due to the ability of iPSCs to differentiate through the neuronal lineage. iPSCs derived from a donor with one of these neurodegenerative diseases and its associated genetic mutation can be genetically corrected through homologous recombination, ZFN, TALEN, or Crispr/Cas9 [5]. These modified cells can then be transplanted back into the patient where they can reproduce into an ample number of healthy cells. It has also been shown that multipotent neural crest stem cells can be derived from iPSCs. These neural crest stem cells promote nerve regeneration by differentiating into Schwann cells and aiding in the myelination of axons [10].
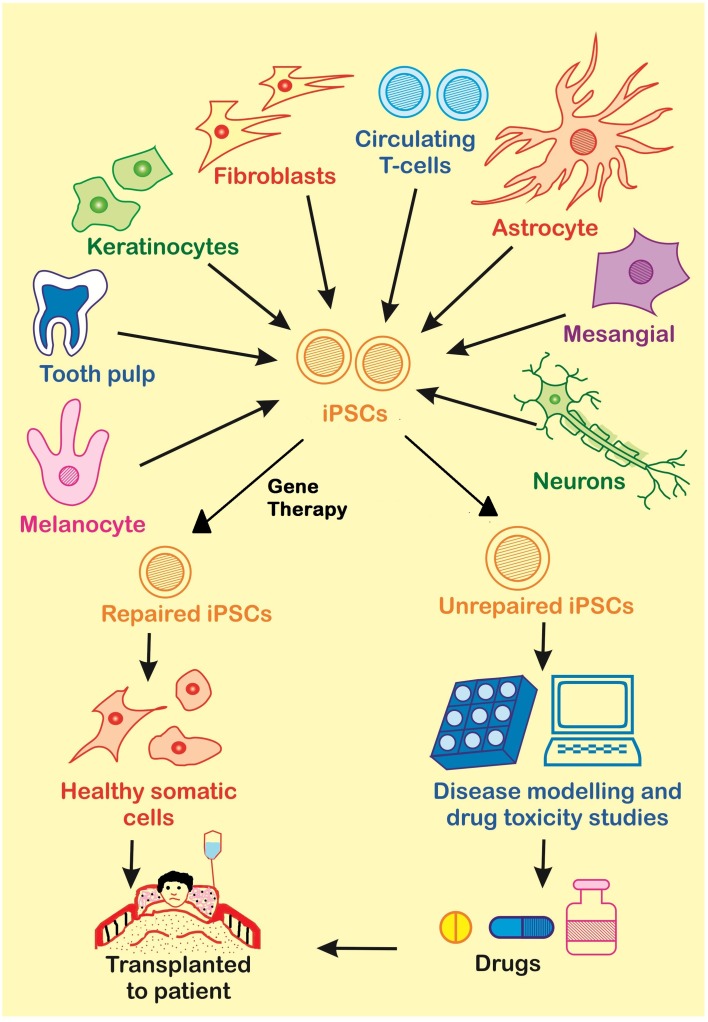
Spinal Cord Injuries
The spinal cord is a collection of nerves that transmits signals between the body and the brain. Spinal cord injuries are extremely serious as they can cause paralysis of extremities, the inability to breathe or speak, loss of control over the bladder, and more depending on the seriousness and location of the injury [6]. Some currently used treatments include medicines, physical therapy, braces, and surgery, but tissue regeneration provides an alternative as it can restore function of the essentially non-regenerative central nervous system. iPSCs have been used to create neural progenitor cells using bovine serum to induce differentiation [6]. These neural progenitor cells can be used to maintain the important role the central nervous system plays in the body.
Retinal Disease
The retina is a layer of tissue that is sensitive to light and relays nerve impulses to the brain to create an image [4]. Diseases that affect the function of the retina include age-related macular degeneration, retinitis pigmentosa, and Stargart’s disease. Tissue regeneration is a powerful competitor in the list of therapies for retinal disease due to the accessibility of the eye, the extensive surgical knowledge regarding the retina, and the immune protection provided by the blood-retina barrier [4]. By treating iPSCs with Wnt and Nodal antagonists, retinal pigment epithelium progenitor cells have been successfully created which allows for the opportunity of transplantation therapies [3].
Clinical Trials
The first clinical trial that utilized iPSCs to regenerate tissue in humans occurred in Kobe, Japan in 2014 and was undertaken by a team of optometrists and surgeons led by Yasuo Kurimoto. In this trial a sheet of retinal pigment epithelium cells was transplanted into a Japanese woman who suffered from age-related macular degeneration, a condition that causes disintegration of the retinal epithelium [13]. This can result in blindness. The trial was conducted at the Institute for Biomedical Research and Innovation and began with creation of iPSCs that could differentiate into the necessary retinal pigment epithelium cells [13]. After the surgery it was noted that the patient did not experience any severe medical problems or bleeding. The clinical trial was then halted by the Riken Center for Developmental Biology (CDB) due to an irregularity found in the iPSCs. These trials are expected to resume in 2017 [7].
Advantages
Potential and evident advantages of iPSCs include:
- Their ability to differentiate into any type of cell regardless of their origin cell type [7]
- The abundancy of their adult somatic cell precursors within the body [7]
- Their elimination of ethical concerns posed by ESCs, such as the death of the embryo during their isolation [7]
- Immune compatibility between donor cells and the patient [12]
- The generation of almost unlimited amounts [1]
- The study of diseases in vitro [9]
- The testing of experiments in a human model without endangering the health of any person [12]
- The reduction of cost during pre-clinical trials due to elimination of animal testing [12]
Disadvantages
Current disadvantages and/or challenges surrounding iPSCs that must be overcome in order for them to be properly used in the medical field in the future include:
- The minimal effectiveness of the technology used to create iPSCs [14]
- Mutagenesis from viral vectors and incorporation of their genetic material into the iPSCs [14]
- Cancer development from the overexpression of oncogenes in iPSCs [12]
- The development of diseases due to enhancement molecules used during iPSC production [17]
- Their low reprogramming rate [14]
- Premature ageing and apoptosis of the iPSCs [1]
- A decrease in the ability of the cell to repair its DNA [14]
- Incompleteness of reprogramming [12]
References
[1] Li, J., Song, W., Pan, G., & Zhou, J. (2014). Advances in understanding the cell types and approaches used for generating induced pluripotent stem cells. Journal of Hematology & Oncology, 7(50). <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4445637/>
[2] Ahmed Al-Anazi, K. (2015). Induced Pluripotent Stem Cells and Their Future Therapeutic Applications in Hematology, 5(1). <http://dx.doi.org/10.4172/2157-7633.1000258>
[3] Hirami, Y., et al. (2009). Generation of retinal cells from mouse and human induced pluripotent stem cells. Neuroscience Letters, 458(3), 126-131. <http://dx.doi.org/10.1016/j.neulet.2009.04.035>
[4] Ramsden, C., Powner, M., Carr, A., Smart, M., da Cruz, L., & Coffey, P. (2013). Stem cells in retinal regeneration: past, present and future. The Company of Biologists Ltd, 140(12), 2576-2585. <http://dev.biologists.org/content/develop/140/12/2576.full.pdf>
[5] Ross, C. A., & Akimov, S. S. (2014). Human-induced pluripotent stem cells: potential for neurodegenerative diseases. Human Molecular Genetics, 23(R1), R17–R26. <http://doi.org/10.1093/hmg/ddu204
[6] Nakamura, M., & Okano, H. (2013). Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Research, 23(1), 70–80. <http://doi.org/10.1038/cr.2012.171>
[7] Scudellari, M. (2016). How iPS cells changed the world. Nature, 534(7607), 310–312. <http://www.nature.com/news/how-ips-cells-changed-the-world-1.20079>
[8] Liu, Z., Zhou, J., Wang, H., Zhao, M., & Wang, C. (2013). Current status of induced pluripotent stem cells in cardiac tissue regeneration and engineering. Regenerative Medicine Research, 1(1), 6. <http://doi.org/10.1186/2050-490X-1-6>
[9] Martins, A. M., Vunjak-Novakovic, G., & Reis, R. L. (2014). The Current Status of iPS Cells in Cardiac Research and Their Potential for Tissue Engineering and Regenerative Medicine. Stem Cell Reviews, 10(2), 177–190. <http://doi.org/10.1007/s12015-013-9487-7>
[10] Wang, A., Tang, Z., Park, I.-H., Zhu, Y., Patel, S., Daley, G. Q., & Song, L. (2011). Induced Pluripotent Stem Cells for Neural Tissue Engineering. Biomaterials, 32(22), 5023–5032. <http://doi.org/10.1016/j.biomaterials.2011.03.070>
[11] Truskey, G. A. (2016). Advancing cardiovascular tissue engineering. F1000Research, 5, F1000 Faculty Rev–1045. <http://doi.org/10.12688/f1000research.8237.1>
[12] Singh, V. K., Kalsan, M., Kumar, N., Saini, A., & Chandra, R. (2015). Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in Cell and Developmental Biology, 3, 2. <http://doi.org/10.3389/fcell.2015.00002>
[12] Medvedev, S., Shevchenko, A., & Zakian, S. (2010). Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta Naturae, 2(5), 18-27. <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3347549/pdf/AN20758251-05-018.pdf>
[13] Cyranoski, D. (2014). Japanese woman is first recipient of next-generation stem cells. Nature. <http://www.nature.com/news/japanese-woman-is-first-recipient-of-next-generation-stem-cells-1.15915>
[14] Goldthwaite, C. (2016). The Promise of Induced Pluripotent Stem Cells (iPSCs). The National Institutes of Health. < //stemcells.nih.gov/info/Regenerative_Medicine/2006Chapter10.htm>
[15] Takahashi, K., & Yamanaka, S. (2006). Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell, 126(4), 663–676. <http://dx.doi.org/10.1016/j.cell.2006.07.024>
[16] Stadtfeld, M., & Hochedlinger, K. (2010). Induced pluripotency: history, mechanisms, and applications. Genes & Development, 24(20), 2239–2263. <http://doi.org/10.1101/gad.1963910>
[17] Menon, S., Shailendra, S., Renda, A., Longaker, M., & Quarto, N. (2016). An Overview of Direct Somatic Reprogramming: The Ins and Outs of iPSCs. International Journal of Molecular Sciences, 17(1), 141. <http://doi.org/10.3390/ijms17010141>
