IGEM:Peking/2007/Count-Conjugation-Notebook/2007-8-9
From OpenWetWare
Jump to navigationJump to search
- First, I have to say sorry because I upload the previous experiment records to our notebook so late. But late is ever better than never, right. n_n--Tao Yu 08:00, 9 August 2007 (EDT)
Tandem Ori-T byMingzhi Qu, Ze Ren
2007-8-8 conjugation failure: Testing
- test why R751 X Dh5α+pSB1A2(amp+) can grow on (Tc+/Amp+) & Dh5α+psc101(Tc+) X Dh5α+pSB1A2(amp+) can grow on (Tc+/Amp+).
colony PCR Test for R751, pSC101(I)
- Test Lb- R751 plate, Lb- pSC101 have the correct plasmid.
- Test plate:LB- R751, Lb- pSB101, Amp+ Dh5α-R0040(as control), R751 plasmid, pSC101 plasmid.
- primer :R751 OriT primer, pSC101 primer.
- according to <Count:Colony PCR STANDARD PROTOCOL>
- PCR system contains(each well):
0.5 µl Primer 1(100uM) 0.5 µl Primer 2 2 µl dNTP(2.5uM) 2.5 µl 10X Taq Buffer 0.25 µl Taq 19 µl dH20 1 µl template -------------------------- ~25 µl Total
- PCR program condition 1: 94℃ 5min, 94℃ 30s, 51℃ 30s, 72℃ 45s, Go to step 2 for 29 times, 72℃ 10min, 4℃ end.
- PCR program condition 2: 94℃ 5min, 94℃ 30s, 55℃ 30s, 72℃ 45s, Go to step 2 for 29 times, 72℃ 10min, 4℃ end .
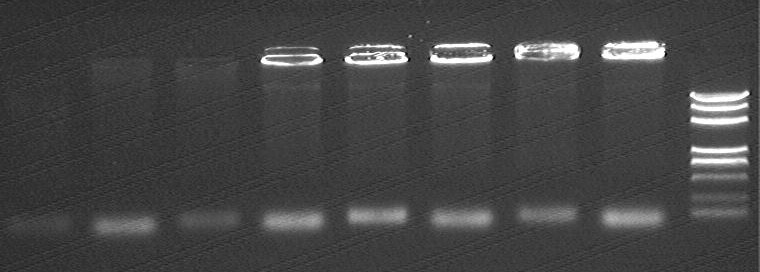
electrophorsis result
- from left to right
- Dh5α-R0040 @ pSC101 Primer
- Dh5α-R0040 @ R751 Primer
- R751 @ pSC101 Primer
- R751 @ R751 Primer
- pSC101 @ pSC101 Primer
- pSC101 @ R751 Primer
electrophorsis result(control)
- from left to right:
- pSC101 plasmid PCR product @Primer vf2,vr
- R751 genome PCR product @Primer vf2,vr
mini-prep F-OriT_J23066_OriT_pSB1A2(normal & fast T4)
- using Transgen mini plasmid puriflication kit.
- 30µL after purflication.
mini-prep double digesting test
- Digesting F-OriT_J23066_OriT_pSB1A2 with EcoRI/PstI.
- F-OriT_J23066_OriT_pSB1A2 Digestion system contains:
1 µl 10*H 0.25 µl EcoRI 0.25 µl PstI 5 µl Plasmid 3.5 µl dH20 -------------------------- 40 µl Total
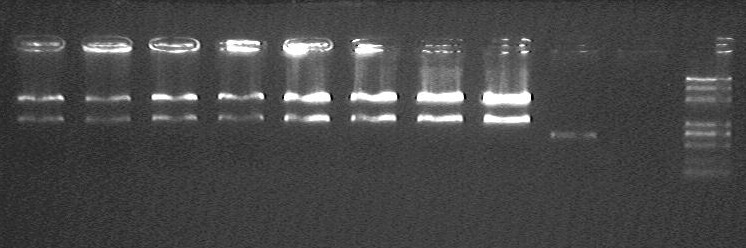
electrophoresis result
- from left to right:
- F-OriT_J23066_OriT_pSB1A2 fast T4-1 @ EcoRI/PstI
- F-OriT_J23066_OriT_pSB1A2 fast T4-2 @ EcoRI/PstI
- F-OriT_J23066_OriT_pSB1A2 fast T4-3 @ EcoRI/PstI
- F-OriT_J23066_OriT_pSB1A2 fast T4-4 @ EcoRI/PstI
- F-OriT_J23066_OriT_pSB1A2 normal T4-1 @ EcoRI/PstI
- F-OriT_J23066_OriT_pSB1A2 normal T4-2 @ EcoRI/PstI
- F-OriT_J23066_OriT_pSB1A2 normal T4-3 @ EcoRI/PstI
- F-OriT_J23066_OriT_pSB1A2 normal T4-4 @ EcoRI/PstI
- OriT_J23066_pSB1A2 @ EcoRI/PstI
- pSB1A2
- Marker(DL2000 plus)
Lock & Key by Yu Tao
Key1 & Lock 1 Efficiency Test
- Add 1 mL overnight incubated sample culture of each group to 9 mL fresh LB.
- Incubate them in 37℃ for 2 hours.
- Divide the R0010.J23066(Key) and R0040.J23078.E0040.B0015-DH5a into 2 test tubes, add IPTG(finally 1uM) to only one of the two.
- Culture all the samples overnight.