IGEM:Peking/2007/Count-Conjugation-Notebook/2007-8-17
From OpenWetWare
Jump to navigationJump to search
Tandem OriT by Qu Mingzhi, Ren Ze
mini-prep R-OriT, S-Orit (T-vector)
- using Transgen mini plasmid puriflication kit.
- 50µL after purflication
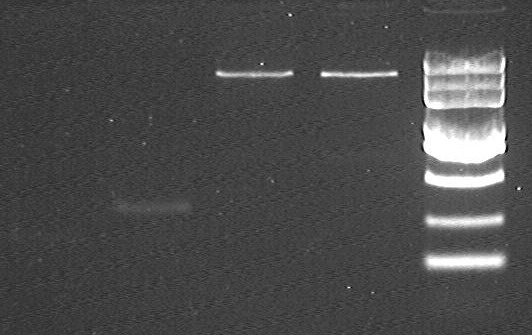
Double digesting test for R-OriT, S-OriT, E0040
- use Double digesting test for gel extraction.
- R-OriT digestion system contains(Standard Assembly fragment):
4 µl 10*H buffer 1 µl EcoRI 1 µl PstI 20 µl Plasmid 14 µl dH20 -------------------------- 40 µl Total
- S-OriT digestion system contains(Standard Assembly fragment):
4 µl 10*H buffer 1 µl EcoRI 1 µl PstI 20 µl Plasmid 14 µl dH20 -------------------------- 40 µl Total
- E0040 digestion system contains(Standard Assembly vector):
4 µl 10*H buffer 1 µl EcoRI 1 µl PstI 20 µl Plasmid 14 µl dH20 -------------------------- 40 µl Total
electrophoresis result before gel extraction
- from left to right:
- 1-2 S-OriT @ EcoRI/PstI
- 3-4 R-OriT @ EcoRI/PstI
- 5-8 pSB1A2 @ EcoRI/PstI
- 9 Maker (DL2000 plus)
electrophoresis result after gel extraction
- from left to right:
- S-OriT
- R-OriT
- pSB1A2
- pSB1A2
- Maker (DL2000 plus)
Ligation: S-OriT -> pSB1A2, R-OriT -> pSB1A2
- Ligate the S-OriT fragment and pSB1A2 vector .
- Ligate the S-OriT fragment and pSB1A2 vector .
- use super fast T4 DNA ligase
super fast T4 DNA ligase Ligation system contains:
1 µl vector 7 µl E0240 fragment 0.5 µl ''super fast T4 DNA ligase'' 1 µl 10X T4 ligase buffer 0.5 µl dH20 -------------------------- 10 µl Total
transformation S-OriT -> pSB1A2, R-OriT -> pSB1A2
NEXT DAY: received 50+ clones, self-ligation did not grow.
Amplification Culture of PlacI-I741051 <- E0240, R0010 <- E0240
select Positive PlacI-I741051-E0240_pSB1A2, R0010-E0240_pSB1A2 Colonies from Plate, Culture in liquid LB, waiting for mini-prep.
Lock & Key By Yu Tao
Mini-prep: T-J01008 and R0010.J01008<-B0015
- Using Transgen mini plasmid purification kit.
- 50uL per tube after purification, 2 tubes per type of plasmids.
Mini-prep Double Digesting Test Result
- Digesting all plasmids above and R0010.J01008(as negative control) with EcoRI/PstI.
- Each digestion system contains:
1 µl 10*H buffer 0.25 µl EcoRI 0.25 µl PstI 3 µl Plasmid 5.5 µl ddH20 -------------------------- 10 µl Total
- 37℃ culutre for 3 hours.
- from left to right:
- T-J01008-1 @ EcoRI/PstI
- T-J01008-2 @ EcoRI/PstI
- R0010.J01008<-B0015-1 @ EcoRI/PstI
- R0010.J01008<-B0015-2 @ EcoRI/PstI
- R0010.J01008 @ EcoRI/PstI
- marker (DL2000 Plus)
- Conclusion: We can see the J01008 fragment. However, R0010.J01008 fragment band is smaller than 200bp band, which means the previously ligated R0010<-J01008 might be wrong.
R0040.J01010 Digestion Product Purification
- use Transgen Quick Gel Extraction Kit.
- 30uL per tube after purflication, one tube, respectively.
Electrophorsis Result
- from left to right:
- R0040.J01010 @ EcoRI/SpeI purified digestion product
- marker (DL2000 plus)
Ligation: R0040.J01010<-E0040.B0015
- Ligate the R0040.J01010 fragment and E0040.B0015 vector
- Ligation system contains:
7 µl R0040.J01010 fragment 1 µl E0040.B0015 vector 0.5 µl Super T4-Ligase 1 µl 10 X ligation buffer 0.5 µl ddH20 -------------------------- 10 µl Total
- The negative control group contains no fragment but ddH2O instead.
- 10min at 16℃
Transformation: R0040.J01010<-E0040.B0015 ligation product
- Transform all ligation product into 100 µl DH5α competent cells.
- Culture all cells at Amp+ LB plate for 12 hours.
- Result to be seen tomorrow.
Double Digestion: R0040.J01010 and E0040.B0015
- Digesting R0040.J01010 with EcoRI/SpeI and E0040.B0015 with EcoRI/XbaI.
- Each digestion system contains:
For R0040.J01010 For E0040.B0015 2 µl 10*H / 2 µl 10*M 0.5 µl EcoRI / 0.5 µl EcoRI 0.5 µl SpeI / 0.5 µl XbaI 17 µl Plasmid / 10 µl Plasmid 0 µl ddH2O / 7 µl ddH2O ---------------------------------------------------- 20 µl Total / 20 µl Total
- 37℃ overnight.
R0040.J01010 and E0040.B0015 Digestion Product Purification
- Use Transgen EasyPure PCR Purification Kit for E0040.B0015 and Quick Gel Extraction Kit for R0040.J01010.
- 30uL per tube after purflication, one tube, respectively.
Electrophorsis Result
- from left to right:
- E0040.B0015 @ EcoRI/XbaI purified digestion product
- R0040.J01010 @ EcoRI/SpeI purified digestion product
- R0040 @ EcoRI/PstI
- R0040.J01010 @ EcoRI/PstI
- marker (DL2000 plus)
Mini-prep Preparation: R0010, R0040.J01010 and E0040.B0015
- Culture positive colonies in liquid LB overnight for mini-prep.