IGEM:IMPERIAL/2006/Protocols/T9002
| Actual Part |  Link to Project Browser (Test Sensing Prey Construct) | ||
|---|---|---|---|
<bbpart>T9002</bbpart>: Transfer Function Characterisation & <bbpart>J37016</bbpart>/<bbpart>J37020</bbpart> Predator Cell Test Construct
Motivation
The motivation behind this <bbpart>T9002</bbpart> test construct is to characterise the transfer function linking AHL input to GFP output.
This will allow us to construct a standard transfer function curve which will allow us to relate GFP output to an unknown AHL input, forming the basis of an AHL assay.
We also would like to explore the AHL input to GFP output in our predator cell constructs <bbpart>J37016</bbpart> and <bbpart>J37020</bbpart> (as it will directly link to AiiA and LuxR production in the predator cell).
Materials & Equipment
- Equipment
- Wallac Victor 3 Multi-Well Fluorimeter
- Eppendorf Tubes
- Small White Cap Tubes
- Large White Cap Tubes
- Gilson Pippettes
- 37°C Shaker
- T9002/J37016/J37020 Report Sheet
- Materials
- Dilution series of AHL [1000 μM, 100 μM, 10 μM, 5 μM, 1 μM, 500 nM, 100 nM, 50 nM, 10 nM, 1 nM]
- GFP Standard Solution
- LB Medium with 40 μg/mL Ampicilin
- E.coli DH5a Culture Containing T9002/J37016/J37020
Protocol
<showhide>
- Inoculate a culture from 10ul of stored T9002/J37016/J37020 in 2ml LB medium containing 50 μg/mL Ampicillin.
- Incubate at 37°C for overnight in a shaker. __HIDER__
<hide>
- This is to get a good stock of cells for use in the experiment. After the overnight culture the cells will be in stationary phase
</hide> </showhide>
- In the morning, prewarm 70 mL LB Amp medium in the 37°C water bath.
- Measure and record OD600
<showhide>
- Inoculate a 16ml fresh culture from the o/n to bring back the OD600 to 0.1, use the prewarmed LB + Ampicilin in waterbath. __HIDER__
<hide>
- Using prewarmed LB/M9 prevents a temperature shock to the culture, which would increase lag time
</hide> </showhide>
\frac{0.1}{\mbox{OD of culture}} \times \mbox{16 mL}
</amsmath>- OD of T9002/J37016/J37020 culture (1st Measurement): _________
- Amount to dilute of T9002/J37016/J37020 culture = ________ mL (amount of original culture to use)
- Amount of prewarmed LB with Ampcilillin to use = ________ mL (16 mL - above result)
- Return LB to 37°C waterbath
<showhide>
- Incubate new culture at 37°C for 2 hours in a shaker __HIDER__
<hide>
- This returns cells to exponential phase from stationary phase
</hide>
</showhide>
After 2hrs in the shaker:
- After the 2 hours measure and record the OD600
<showhide>
- Dilute again for an OD of 0.1 in a new culture of 25ml of a prewarmed LB + Ampicilin. __HIDER__
<hide>
- This dilution gives a standard OD to which to innoculate the culture with AHL (in this case 0.1). Innoculating at different ODs is known to give different results, so it is important a standard OD is used
</hide> </showhide>
- <amsmath>
\frac{0.1}{\mbox{OD of culture}} \times \mbox{25 mL} </amsmath>
- OD of T9002/J37016/J37020 culture (1st Measurement): _________
- Amount to dilute of T9002/J37016/J37020 culture = ________ mL (amount of original culture to use)
- Amount of prewarmed LB with Ampcilillin to use = ________ mL (25 mL - above result)
- Vortex new T9002/J37016/J37020 culture.
- To start AHL incubation:
- Label each tube with AHL concentration
- Put 20ul of the AHL into 11 seperate 5ml white capped tubes
- Add appropriate amount of T9002 samples as per the table below
- Record time of inoculation in report sheet.
- Vortex each tube
<showhide>
- Incubate all 5mL tubes in a 37°C shaker for 4 hours so GFP expression can reach steady state __HIDER__
<hide>
- Do not pipette the samples into the 96 well plate yet - because if the 96 well plate is put in the shaker, cross-contamination between the well is very likely to happen.
</hide></showhide>
|
After 4hrs in the shaker:
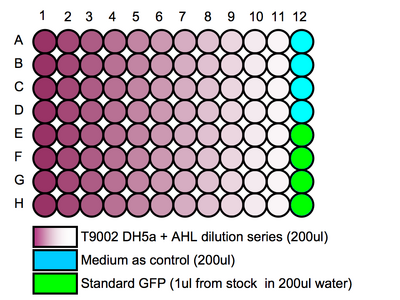
- Add a 200uL sample from each eppendorf tube to the 96 well plate.
- Do this for 8 repeats following suggested patterning (see above)
- NOTE: Since all repeats are made from the same culture, it is enuogh to do 4 repeats - thus one plate can be used for 2 different tests. (Pipetting errors will be ruled out since absorbance is measured later and can then be considered when processing the data.)
- Add 4 x 200uL of growth medium to a well to act as a control.
- Take the plate and eppendorfs to BCHEM
<showhide>
- Add 995ul of ultra pure water to the eppendorf, together with 5ul of undiluted GFP standard solution and mix __HIDER__
<hide>
- A 200 x dilution of GFP is made.
- The undiluted GFP is in the BCHM Level 6 Cold Room on our shelf. It is in a small grey plastic box. Pippettes, tips and ultrapure water are located on a shelf above the electroporation machine in the plate reader room.
</hide> </showhide>
- Add 4 x 200uL of the 200x diluted GFP standard solution to the wells following the suggested patterning
- Take a reading
- Take the plate to the plate reader room
<showhide>
- Use the Victor3 to measure flourescence and absorbance __HIDER__
<hide>
- Use the preprogrammed Assay under the 'Students' folder called GFP + Abs490
</hide> </showhide> <showhide>
- Repeat the measurment a further two times straight after each other __HIDER__
<hide>
- This to assess the variability of the machine
</hide> </showhide> <showhide>
- Save data file from computer. __HIDER__
<hide>
- You'll need a memory stick to save the information to. Unfortunatley the computer isn't networked
</hide> </showhide>
- Copy and paste the data into a T9002/J37016/J37020 Data Spreadsheet
Last Updated: Tom 15:06, 18 August 2006 (BST)
Last Updated: Farah 04:09, 22 August 2006 (EDT) (changes made: changed 30ml culture to 25ml and amended volume of sample concentration (it previously read 1880ul)).
Last Updated: Johnsy 05:59, 22 August 2006 (EDT)
Last Updated: Christin 13:24, 12 September 2006 (EDT)
Many thanks to Drew Endy and Barry Canton from MIT for providing the protocol on which this is based.
Potential Issues
- What is the GFP standard solution? Do we know its concentration? How stable is that? half life?
- JS: Please can you write a protocol that will decouple other experiments with this one in case we want to assay an unknown concentration of AHL. Thanks. (i.e. take an unknown concentration of AHL of x amount into the T9002....measure flourescence...etc)