IGEM:IMPERIAL/2009/Encapsulation/Phase2/Alginate Properties
Alginate Properties
Currently produced by brown seaweed (algal)
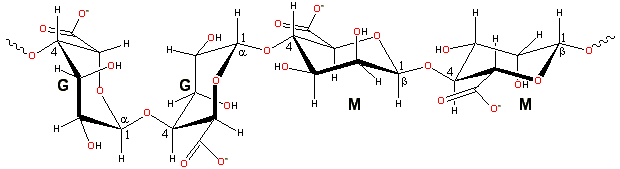
linear unbranched polymers containing β-(1 4)-linked D-mannuronic acid (M) and α-(1 4)-linked L-guluronic acid (G) residues
Uses
Ca-alginate is commonly used for the entrapment of biocatalysts, especially whole cells for the inoculation of soil
, the degradation of surfactants and pesticides in waste water
or the production of food stuff, industrial enzymes and fine chemicals
Biochemistry of structure
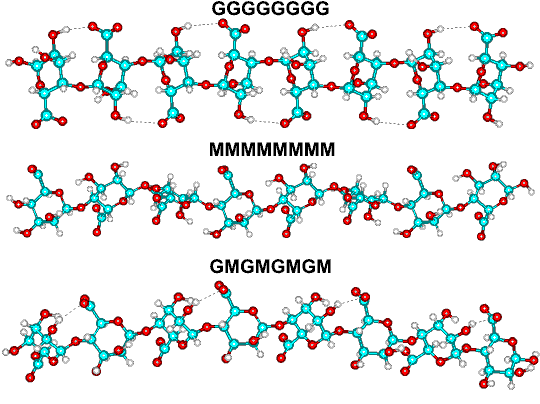
M prefers forming a 3-fold left-handed helix with (weak) intra-molecular hydrogen bonding between the hydroxyl group in the 3
position and the subsequent ring oxygen (that is, O3-H O').
G forms stiffer (and more acid-stable) 2-fold screw helical chains, preferring intra-molecular hydrogen bonding between
the carboxyl group and the 2-OH group of the prior residues and (weaker) the 3-OH group of the subsequent residues. The
diaxial links also inherently allow less flexibility.
The free carboxylic acids (without counter ion) have a water molecule H3O+ firmly hydrogen bound to carboxylate (pKa M 3.38,
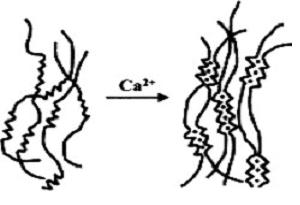
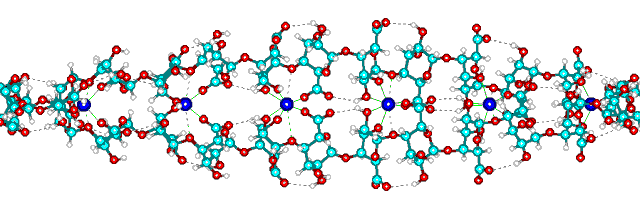
pKa G 3.65). Ca2+ ions can replace this hydrogen bonding, zipping guluronate, but not mannuronate, chains together
stoichiometrically in a supposedly egg-box like conformation
Control
- by controlling ratios of G & M monomers in alginate
- 5-epimerization of β-(1 4)-linked D-mannuronic acid residues to α-(1 4)-linked L-guluronic acid residues in algal alginates
using bacterial epimerases
High G content produces strong brittle gels with good heat stability (except if present in low molecular weight molecules) but
prone to water weepage (syneresis) on freeze-thaw, whereas high M content produces weaker more-elastic gels with good
freeze-thaw behaviour