IGEM:IMPERIAL/2009/Encapsulation/Modelling/Timer1
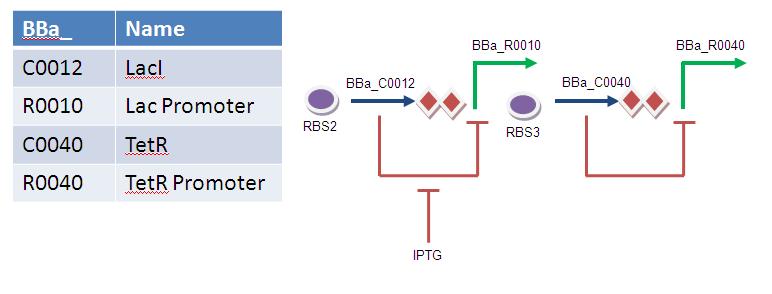
Diagram
Operations
In order to switch on encapsulation, we need to be able to repress tetR. By introducing arabinose, we trigger the production of LacI. Provided that LacI is high enough, TetR is repressed to different extents.
David Roche 10:21, 24 August 2009 (EDT) : From diagram it is not clear of the function of tetR and arabinose. Perhaps label them tet repressible etc?
Table 1. Summary of operation of timer 1
- thresh1 = Upper limit of IPTG within tuneable range
| Arabinose input | State of TetR | IPTG input | Output |
| 0 | 1 | 0 | OFF |
| [ara]>thresh = 1 | 0 (sufficiently repress) | 0 | ON |
| 1 | Not fully repressed | [IPTG]>0, [IPTG]<thresh1* | ON (tunable range) |
| 1 | 1 (not repressed) | [IPTG]>0, [IPTG]>thresh1* | OFF |
Model
[arabinose] - Concentration of arabinose, which is the input
dm1 - Rate of degradation of mRNA 1
[m1] - Concentration of mRNA 1
[p1] - Concentration of protein 1 (LacI)
[kp1] - Rate of translation of mRNA 1 to LacI
dp1 - Rate of degradation of LacI
dm2 - Rate of degradation of mRNA 2, the mRNA coding for TetR
[m2] - Concentration of mRNA 2
[p2] - Concentration of TetR
kp2 - Rate of translation from mRNA 2 to TetR
dp2 - Rate of degradation of TetR
dm3 - Rate of degradation of mRNA 3, the mRNA coding for protein 3
[m3] - Concentration of mRNA 3
[p3] - Concentration of protein 3
kp3 - Rate of translation from mRNA 3 to protein 3
dp3 - Rate of degradation of protein 3
IPTG - Concentration of IPTG added
Simulations
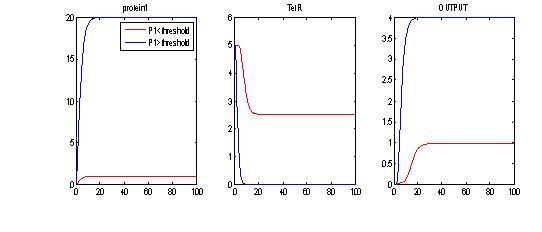
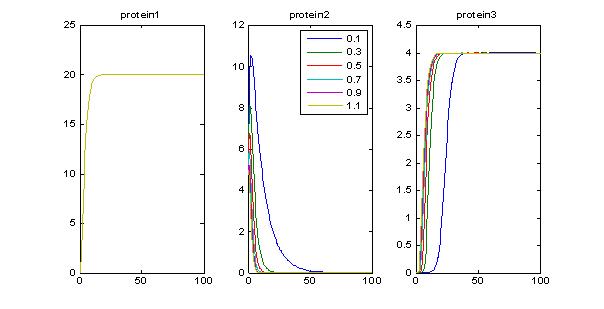
- Investigating the effects of arabinose input: Arabinose must be greater than a given threshold to achieve maximal encapsulation. Otherwise, we partially repress TetR and we get some encapsulation.
- Vary degradation rate of TetR: Now, if we are assuming that [ara]>threshold, we can see the effects of TetR degradation in setting off delays in encapsulation. Ideally we might not want TetR to be too stable.
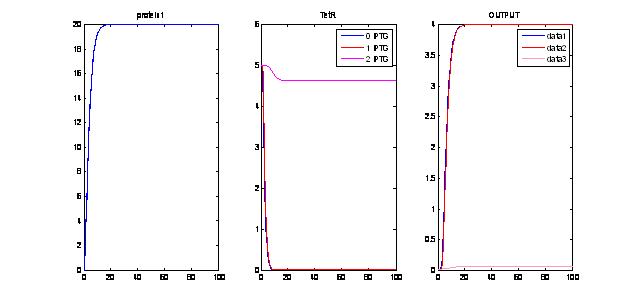
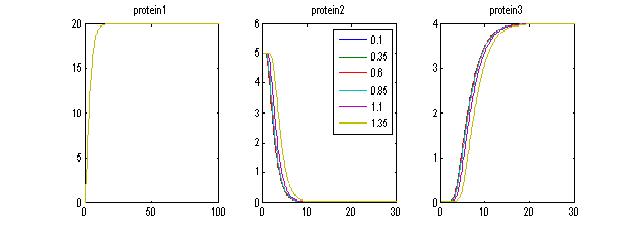
- Assuming there is enough [ara], when we vary IPTG:For P1> Threshold to produce maximal encapsulation, we can see again that for IPTG= 0 AND IPTG=1, we get some encapsulation. For IPTG=2, we “derepress” TetR and therefore, we inhibit encapsulation. This shows that we have a “working tunable range for IPTG”. Working range for IPTG: when IPTG is within a given range (i.e. 0<IPTG<1.5) we can observe sufficient repression of TetR and a bit of delay. However, when IPTG is too much, the LacI-TetR pathway is “de-repressed” too much, therefore, we do not get encapsulation (pink line, OUTPUT)
- IPTG within the “tunable range” produces delays:As we can see, provided that IPTG concentrations are within a “narrow range” we can produce some delays in the encapsulation process. The disadvantage is that the range may be too narrow.
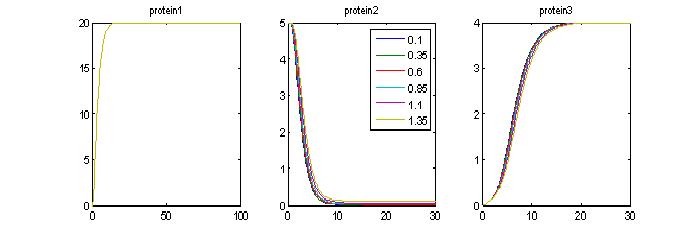
- Effect of lower hill exponent (n): By modifying the hill exponent we may be able to see more of a delays (previously n =5). Now set n=1.5. Ideally we dont want a very steep hill function because the timer will be more of a switch than something tunable.
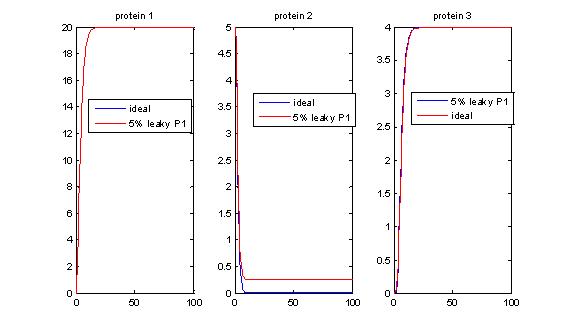
- Effects of promoter leakiness (P1-LacI): Promoter leakiness affects encapsulation yield.
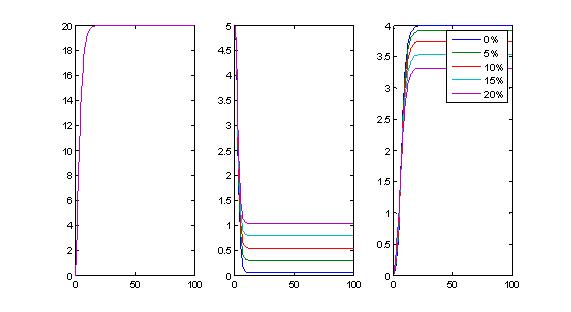
- Effects of promoter leakiness (P2-TetR): If we make the 2nd promoter leaky, we affect the extent of repression of TetR and the encapsulation yield, not necessarily the delays.
- Effects of promoter leakiness (P3-Encapsulation protein):If we make the output promoter leaky , encapsulation occurs quicker. This could be undesirable.
Recommendations
- In order to obtain sufficient output, we must ensure that the levels of LacI (protein1) reach steady state. This is done by introducing sufficient [arabinose].
- The degradation term of TetR will affect the length of the delay in encapsulation. We don’t want the degradation rate to be too low, otherwise encapsulation will take a long time.
- IPTG “derepresses” the LacI, TetR pathway and “tunes” the delays in encapsulation. This only happens for IPTG<some threshold. If the levels of IPTG are too high, we will eventually stop repressing TetR and the encapsulation won’t work. So the range of tunability is very narrow.
- Promoter leakiness: P1 leakiness does not significantly affect delays in encapsulation, but may limit the output yield. Promoter 2 leakiness affects the output levels of protein 3, and promoter 3 leakiness makes the encapsulation process to occur quicker.
Ideally, we do not want our promoters to be leaky. If they are leaky, it is more desirable for P1 and P2 to be leaky (and we can compensate by putting in enough arabinose), but if P3 is leaky this is a problem, as we trigger encapsulation earlier and timer properties are destroyed.
Therefore, it is essential that P3 is as non-leaky as possible
- In general, it is better to rely on activation mechanisms, rather than repression