IGEM:IMPERIAL/2009/Encapsulation/Applications/Anthocynin
Introduction
Anthocyanin are believed to have anti-oxidant properties and hence, anti-cancer properties. Their anti-cancer properties have been intensively researched on and many papers have proven that anthocyanin indeed inhibits cancer growth.
Summary of papers showing anti-cancer properties of anthocyanin, in particular, cyanidin
• Result in a 60% growth inhibition of human HT-29 colon cancer cells by a cytostatic inhibition of cell growth
2. Induction of apoptosis in cancer cells by Bilberry (Vaccinium myrtillus) and the anthocyanins [1]
• Anthocyanins induce apoptosis in cancer cells
• Growth of HL60 human leukemia cells and HCT116 human colon carcinoma cells are inhibited in vitro
• More HL60 apoptosis is observed
• Anthocyanins extracted from bilberry decreased the number of viable HL60 cells by 84% and 88% at a concentration of 4 and 6 mg/mL respectively, and the number of viable HCT116 cells by 66% and 97% at 2 and 4 mg/mL respectively
3. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells [2]
• Anthocyanins and cyanidin reduced cell growth of human colon cancer cell lines HT 29 and HCT 116
• Mice consuming anthocyanins e.g. cyanidin, has smaller (in volume) adenomas in the cecum than mice consuming the control diet.
4. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice [3]
• improves hyperglycemia and insulin sensitivity due to the reduction of retinol binding protein 4 (RBP4) expression in type 2 diabetic mice
More papers to look out for:
Great reference website on anti-cancer properties of anthocynin
- Katsube N, Iwashita K, Tsushida T, Yamaki K, and Kobori M. Induction of apoptosis in cancer cells by Bilberry (Vaccinium myrtillus) and the anthocyanins. J Agric Food Chem. 2003 Jan 1;51(1):68-75. DOI:10.1021/jf025781x |
- Sasaki R, Nishimura N, Hoshino H, Isa Y, Kadowaki M, Ichi T, Tanaka A, Nishiumi S, Fukuda I, Ashida H, Horio F, and Tsuda T. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007 Dec 3;74(11):1619-27. DOI:10.1016/j.bcp.2007.08.008 |
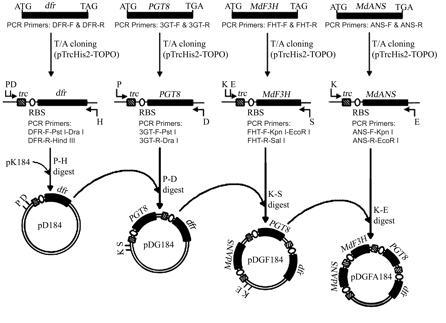
Biosynthesis of cyanidin in E. coli
Pelargonidin 3-O-glucoside and cyanidin 3-O-glucoside were synthesized. We are more interested in cyanidin here.
Biosynthesis pathway: chalcone → flavanone → dihydroflavonol → anthocyanidin → anthocyanin
Problems
1. Low amounts of cyanidin 3-O-glucoside ie 6.0 μg/liter were synthesized.
2. Certain metabolic steps involved are catalyzed by cytochrome P450 monooxygenases, such as flavonoid 3′-hydroxylase and flavonoid 3′,5′-hydroxylase, whose functional expression is challenging
[4]