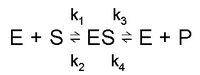IGEM:IMPERIAL/2007/Projects/Modelling/Tutorial
Modelling: Tutorial
Michaelis-Menton Kinetics
Introduction
Michaelis-Menten kinetics describes the kinetics of many enzymes.
Assumptions of Model:
- [Enzyme] is much less than the [Substrate]
- Enzyme is not allosteric.
- Reaction is in steady state
- Equilibrium favours product over transition complex.
Derivation of MM
Consider the following reaction:
k1 and k3 are forward rate constants. k2 and k4 are backward rate constants.
1. Rate of formation of ES: k1[E][S] + k4[E][P]
However, we assume k3 ≫ k4, i.e. the equilibrium favours product over transition complex, so we ignore the term with k4 in it.
2. Rate of removal of ES: k2[ES] + k3[ES]
3. In the steady state, rate of formation of ES = rate of removal of ES:
k1[E][S] = k2[ES] + k3[ES]
If we rearrange this to get all the rate constants on one side and all the concentration terms on the other, we can define a constant called Km, which is the Michaelis constant:
Km = (k2+k3) / k1 = [ES] / [E][S]
4. We can’t measure [E] (free enzyme) or [ES] easily, but we do know the total enzyme concentration, as that is what we will have added when we made up our enzyme solution.
[E] = [E]T – [ES]
If we substitute this into our definition of Km and rearrange it, we get:
[ES] = [E]T[S] / ([S]+Km)
5. The overall (initial) reaction rate V is
V = k3[ES]
If we substitute this into the last result, we get:
V = k3[E]T[S] / ([S]+Km)
6. When the enzyme is saturated, [ES]=[E]T, so we can define a maximum velocity, Vm:
Vm = k3[E]T
And this gives us our final result:
http://upload.wikimedia.org/math/1/3/a/13a6e707391eebde6b0c460f578572a5.png
MM Parameters
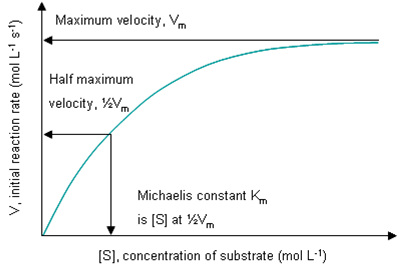
Vm (or Vmax) is the maximum rate at which a certain mass of enzyme can work, and equals k3 (the rate constant for [ES] degradation to product) times the total enzyme concentration [E]T. Fast enzymes have higher Vm. Vm is not a constant, because it is dependent on the amount of enzyme present in a solution, and on the volume of this solution. It is measured in mol s-1 or more usually μmol min-1.
Km is (k2+k3) / k1 = [E][S] / [ES], i.e. Km is the Keq for the non-productive dissociation of the ES complex back to E and S. A low Km indicates the enzyme has a high affinity for its substrate. Therefore efficient enymes have lower Km. Km is a constant: it equals [S] at ½Vm, and is measured in mol L-1, or more usually mM.