IGEM:IMPERIAL/2006/LabCalendar/2006-8-27
From OpenWetWare
Jump to navigationJump to search
To Do:
*Miniprep (J27024 & J37018) and Maxiprep (J27024 & J37018 & 4G(S01656))
*Ligation & Electroporation:
*J37025 = B0034 + J37024
*J37022 = J04500 + J37024
*Testing T9002, J37016, J37020
*Culturing for Monday: for testing T9002, J37016, J37020, J37022
JW: There was a problem on Saturday with the freezing of S01656, in that we did not realise until right at the end of the day that S01656 was Kanamycin resistant. We added ampicillin resistant LB and so the 10 Eppendorfs that are in the -80[[:Category:{{{1}}}|{{{1}}}]] freezer need to be thrown away. I cultured up some new S01656 so that someone could make up some new frozen stocks and this is in the shaker. The kanamycin is in an Eppendorf in the freezer.
Testing of T9002, J37016, J37020
- Took 16mL cultures out of shaker after 2h at 12:40
- OD measurements:
- T9002 0.631
- J37016 0.687
- J37020 0.647
- Dilute down to OD 0.1 into 25mL
- Put 5mL tubes with AHL in shaker at 13:20 - to be taken out at 17:20
- Tubes taken out of shaker at 17:30 and transferred into 96 well plates
- Started plated reading at 20:00 (delayed because of complications with ligations)
Miniprep of J37018 & J37024
- Miniprep successful
- PROBLEM: Gel did not run correctly - perhaps 1*TAE was not concentrated correctly, it might have been too concentrated...
- Miniprep has to be repeated to check the ligations for the above parts
Maxiprep of J37024, J37018, 4G
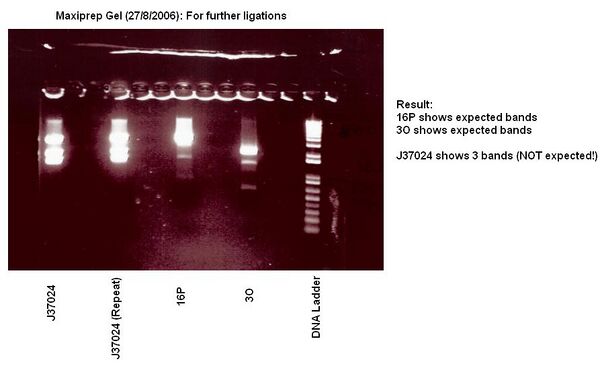
- Maxiprep successful
- We will need to re-maxiprep J37024 and J37018 since looking at the plate has revealed that various colonies seemed to have been cultured....also the colony cultured for miniprep is different to that cultured for maxiprep.
- JW: The maxipreps only contained one colony, and yes, that is different to the miniprep, however if the ligation was successful then it does not matter....... It just means that we do not have a stock of J37024 and J37018, and it would only be for the benefit of the registry to have those.
- CS: Yes, theoretically that would work. However, we cannot assess for sure just saying from one maxiprep and digest for the ligations whether the previous ligations worked since there is a difference of ~100 bp in the un- or successfully ligated parts. Also, the bands that showed up on the gel had band sizes very different from the expected values.
Ligations for J37022 and J37025
- Final Aiia construct: J37022 = 16P + J37024
- For Aiia predator cell: J37025 = 3O + J37024
- Digests of Maxiprep:
- J37022:
- Insert: J37024, cut with X & P (yellow buffer)
- Vector: 16P. cut with S & P (yellow buffer!)
- J37024:
- Insert: J37024, cut with X & P (yellow buffer)
- Vector: 3O, cut with S & P (yellow buffer!)
- J37022:
- Set up 30uL digest
- Leave in 37 C waterbath for 1h (13:00)
- Took out of waterbath at 13:55 and run digests on big gel
- PROBLEM: Gel did not run correctly (even after 1.5h, the wellfronts had hardly moved, at a voltage of 90V) - perhaps 1*TAE was not concentrated correctly, it might have been too concentrated...
- Repeated digests from maxiprep & run on gel again in order to leave to ligate overnight
- BUT: Shock! Horror! - we've realized that only one colony was cultured up for the minipreps today and a 'different' colony was cultured up for the maxiprep and from the gel we ran for the ligations we couldn't really decipher if part J37024 was there or not....so I guess we've lost a day and we'll have to spend tomorrow re-maxipreping and ligating etc.
- Johnsy 18:11, 29 August 2006 (EDT): Yikes...
Culturing for Tomorrow
- T9002/J37016/J37020 for testing (from freezer stock)
- J37018 colonies a-d (for minipreps)
- J37024 colonies a-e (for mini & maxipreps)