IGEM:IMPERIAL/2006/LabCalendar/2006-8-21
From OpenWetWare
Jump to navigationJump to search
- New and Fresh dilution series of AHL: [1000uM, 100uM, 10uM, 5uM, 1uM, 500nM, 100nM, 50nM, 10nM, 1nM]
Maxiprep
- J37031
- J37032
- J37015RS
- 1M B0021
Miniprep
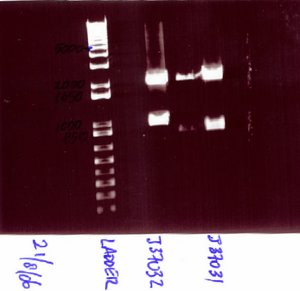
- J37031 & J37032
- Digest with EcoRI & PstI
- 10 μL DNA
- 6 μL ultrapure water
- 2 μL Orange Buffer
- 1 μL each EcoRI & PstI
- J37031 part length = 999 bp, plasmid pSB1AK3 = 3189 bp
- J37032 part length = 938 bp, plasmid pSB1A2 = 2079 bp
- Gel shows two distinct bands for both parts; however, not quite corresponding to the part sizes expected. However, had our ligation not worked, then we would not have gotten the bands that large. We will go ahead and perform the ligation to create J37020 = J37031 + J37032 and sequence afterwards to see if we obtain the part that we desire.
Ligation
- J37020 test construct = J37031 + J37032
- J37031 (vector) cut with SpeI & PstI, expected size = 999 bp + 3189 bp (pSB1AK3) = 4188 bp
- J37032 (insert) cut with XbaI & PstI, expected size = 938 bp
- J27020 miniprep, expected size = 1945 bp (part length) & 3189 (pSB1AK3)
- 30 μL Digest
- 25 μL DNA
- 3 μL Yellow buffer
- 1 μL of each enzyme
PCR
- Performed on the aiiA predator construct as the correct primers eventually arrived today.
- 10 cycles at 49[[:Category:{{{1}}}|{{{1}}}]]
- 20 cycles at 70[[:Category:{{{1}}}|{{{1}}}]]
- Left to run overnight
- Also the LoxP sites are being PCRed overnight
T9002
- Left 96-well plate in shaker overnight to see if steady state is reached.
- Readings to be taken tomorrow.
- Tom 16:13, 21 August 2006 (EDT): Yeah, I know you mentioned it to me earlier on but it didn't really process. This isn't really a good idea. The cells will reach stationary phase overnight, meaning GFP production could be reduced to zero and most likely at least be reduced by a considerable margin.
Cultures
- Cultures set up for testing tomorrow:
- S01656 in 5ml LB KAN (for protein gels)
- 10μLS01656 in 2ml LB KAN (-IPTG)
- 10μL S01656 in 2ml LB KAN (+ 20μL IPTG)
- 10μL T9002 in 2ml LB AMP
- 10μL J37016 in 2ml LB AMP
- 10μL J37015 in 2ml LB AMP
- 10μL J37015RS in 2ml LB AMP
To do Tomorrow
- Glass milk purify DNA from gel.
- Ligate insert into vector (J37032->J37031)
- Electroporate into bacteria
- Continue with AiiA ligations (if immunotag fusion PCR is successful).