HEPES
From OpenWetWare
Jump to navigationJump to search
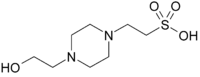
piperazine-N’-
(2-ethanesulfonic acid)
HEPES is a common buffering chemical similar to Tris in Tris-HCl buffers and phosphates in PBS. Buffers are used to keep the pH at a certain value and can "buffer" the addition of small amounts of acids/bases.
Property of HEPES
Stable pH vs. temperature, no primary amine groups, no metal chelation, near physiologic pH range. HEPES is often used to maintain protein solubility in biochemical experiments.
- pKa at 25C of 7.55 (7.31 at 37C); (2nd pKa at pH 3 is not of interest)
- usable buffering range of 6.8 to 8.2
- molecular weight 238.3 g/mol
- ΔpKa/ΔT = -.014
- HEPES contains tertiary amines, which are reactive under certain conditions.
- Chemical formula: C8H18N2O4S; N-(2-hydroxyethyl)-piperazine-N’-(2-ethanesulfonic acid); aka 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; CAS number: 7365-45-9
Buffers are typically 1 M, prepared by neutralizing HEPES with sodium hydroxide. HEPES is essentially insoluble until it is neutralized.
1M HEPES KOH buffer 7.5
- 700 ml ultrapure water
- 238.3 g HEPES
- KOH (potassium hydroxide) pellets to adjust pH to 7.5
- to 1L w ultrapure water; sterilise by filtration
1M HEPES-NaOH pH 7.5
- 700 ml ultrapure water
- 238.3 g HEPES
- ~5.5 g NaOH (sodium hydroxide) pellets to adjust pH to 7.5
- to 1L w ultrapure water; sterilise by filtration
Links
- original publications: Good'66 PMID 5942950, Good'74 PMID 4206745, Blanchard'84 PMID 6717292
- Wikipedia: buffer solution
- CP: common buffers
- List of common buffers from Smith College