Filtration method for competent cells preparation
Introduction
As part of our London Biohackspace iGEM 2015 project we have been working a method for preparing competent cells in an automated and cheap fashion, in order to help other community labs prepare cells easily.
One of the main backslashes of working in a community lab is in fact the difficulty of long term storage of cells as buying and maintaining a -80 freezer is out of the budget of a small lab. We tested the long term storage of glycerol stocks of our constructs in -20, and with regular restreaking it's simple to maintain a library of plasmids in glycerol stocks. The main problem arose when we had to store competent cells, as they in fact very quickly loose competency (days-week).
The only way to have competent cells is to prepare them on the day, which slows down the work and is particularly time consuming. The protocol for preparing electrocompetent cells includes several washing steps, which, if carried out with a centrifuge with a standard methodology can take up to 2-3 hours, not including the preparatory work to initially grow the cells.
We approached this problem with a "hacker" spirit and developed a filtration based method to carry out the washing steps necessary to prepare competent cells. This method is extremely fast (5-10 minutes), automatable and produces highly competent cells. We took inspiration from George Church's MAGE automation platform to carry out this process and some tips on the Electrocompetent_cells page, which utilises a filtration step to make the cells electrocompetent.
The protocol showed below was tested with a syringe filter and yielded good initial results, we are now looking forward to test this further beyond the iGEM project and implement an automated system for our lab.
Materials and methods
Standard protocol for electrocompetent cells preparation
Refer to http://2015.igem.org/Team:London_Biohackspace/protocols/ecoli-tansformation
Filter based method
- Inoculate 1:100 in the morning from overnight colture of DH10b cells
- Once cells are at OD 0.4 move them to ice and keep them ice-cold throughout the process
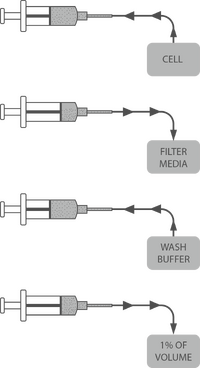
- Take 5ml into a Syringe
- Press 5ml of colture against a 0.22um filter
- Resuspend through the filter in 5ml of ice-cold HEPES 1mM
- Repeat the last two steps 3 times, washing in a total of 15ml of HEPES. If necessary, 50ul of the waste can be electroporated to test for conductivity by looking at the time constant.
- Resuspend through the filter in 250ul
- Spin at 1000g for 3 minutes
- Resuspend in 50ul of HEPES 1mM and it's ready to transform!
Transformation
To test for competency we added 1ul of the part BBa_K554001 at 1ng/ul to the 50ul of cells, left 5 minutes on ice, transferred to prechilled 2mm cuvette and electroporated with a final time constant of 5.05us. 450ul of 2xTY was added and the cells were recovered for one hour at 37 degrees.
We plated 20ul of cells at four different concentration upon serial dilution: 8E3, 8E2, 8E1 and 8E0 cells expected if competency of 10E8 CFU/ng. The results were the following:
3: 10-15 colonies every 20ul
2: 0,2,7 colonies every 20ul
1: 0-1 colonies every 20ul
0: 0,2,2 colonies every 20ul
Results
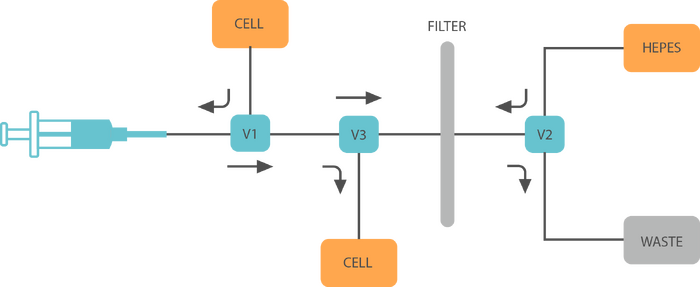
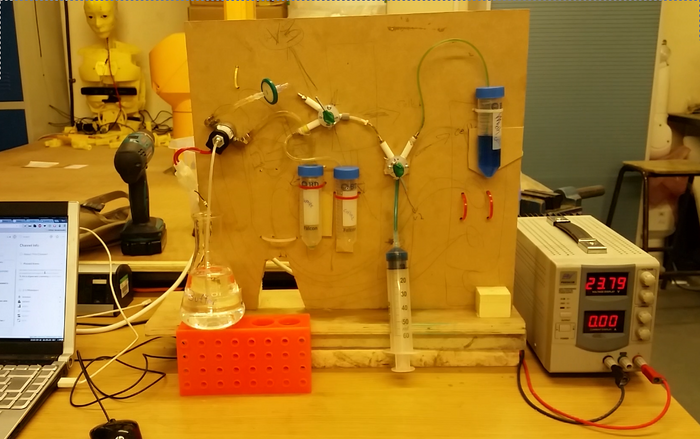
With this initial test we confirmed the results obtained by the Church group and were able to successfuly obtain highly competent cells by avoiding the laborious centrifugation steps and using instead a filtration system. This allows for automation to be easily implemented, and we are currently building a platform to allow a simple competent preparation system. We hope that this method, coupled with an automated machine will allow biohackspaces to easily obtain competent cells without spending hours preparing them when there is no -80 freezer to store them long term. This will hopefully facilitate the expansion of access to synthetic biology techniques in society. The diagram below shows our current prototype that we tested with some food dyes, keep an eye on this page! :)