Electrospun Materials: By Helen Hua and Emma Klinkhamer
Background
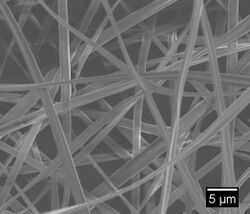
Electrospinning is a simple technique in which a mat is fabricated from nonwoven fibers with diameters ranging from nano to micro sized. The electrospinning technique can be used to spin a variety of natural and synthetic polymers. Electrospun fibers can be used in a wide range of applications ranging from drug delivery, liquid filtration, and tissue engineering. This is a process that utilizes a high voltage source to inject a polymer solution or melt with a specific polarity. The solution is dispensed from the needle tip at a very low feed rate. The fiber jet accelerates toward a collector of opposite polarity to form a mat. Simultaneously a voltage is applied to the needle tip as well as a collection plate, typically around 10 cm away from the needle tip. As the polymer liquid begins to build on the collector, the electrostatic attraction between the polymer and the collector increases. Likewise, the repulsion between the liquid increases. The polymer extends to collection plate in fiber form, while the solvent evaporates. This produces the electrospun fiber mat. This technique can be used to form fibers that are a few micrometers in diameter to tens of nanometers [1].
The electrospinning process has many parameters that can be manipulated in order to fine tune the desired mat, such as polymer concentration, viscosity, and molecular weight [3]. For example, the distance from the collection plate to the needle tip can be increased in order to decrease fiber diameter; spinning time can be increased to create a higher density fiber mat. Additionally there are myriad polymer solution choices. The choice of polymer is very application dependent. For instance, cellulose acetate can be spun to create mats that would optimize wound healing as shown in Figure 1. Similarly, chitosan can be spun in order to create anti-bacterial membranes [2]. Beside a user defined polymer choice, another benefit of electrospinning is the spinning method itself. For example, a lot of research has gone into using a rotating drum to create a tube of fibers. There are some collector mechanisms that even produce an aligned arrangement of fibers as opposed to the non-woven matrix [4].
History
1934-1944: Anton Formhals patents process and apparatus for producing polymer fibers using an electrostatic force [1].
1952: Vonnegut and Neubauer develop apparatus in which a wire is put in contact with liquid contained within a glass capillary tube, thus electrifying liquid droplets [5].
1955: Drozin finds that certain liquids can be dispersed as a uniform aerosol depending on conditions.
1966: Simons patents an apparatus that produces thin and lightweight fibers using two electrodes [5].
1971: Baumgarten creates set-up that produces small diameter fibers using a stainless steel capillary tube, an infusion pump, a high voltage DC current and a metal screen to collect fibers
1970s: Annis and Bornat produced the first electrospun fibers for the use in tissue engineering. The two researchers electrospun polyurethane mats to be as a vascular prosthesis [1].
1994: Reneker coined the term "electrospinning". Before 1994, the process was referred to as "electrostatic spinning" [1].
Main Components
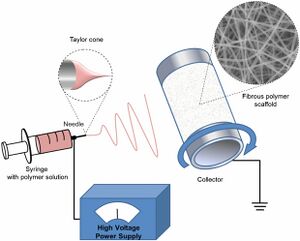
Syringe Pump and Needle
The syringe pump is used to force the polymer solution or melt through the needle. Gravitational forces as well as pressurized gas can be used as well for the syringe pump. The flow rate that forces the liquid through the needle affects the fiber size, porosity and shape [1].
High Voltage Source
The high voltage supply is connected to the needle and injects charge into the polymer liquid. As the voltage increases, the leading edge of the solution forms a cone shape called the Taylor cone. The Taylor cone allows for the formation of fibers that are smaller than the needle in diameter Increasing or decreasing the voltage changes if the Taylor cone forms and where the Taylor cone is in relation to the needle [1].
The fiber jet starts to eject from the surface of the droplet once the applied electric field is strong enough to overcome the surface tension of the polymer [3].
Collector
The collector is typically a metal plate of opposite polarity in order to attract the fibers to the plate. The conductivity and the porosity of the collector can affect the packing density of fibers. The distance between the needle and the collector affects the fiber size. Increasing the distance has found to cause smaller fiber diameters [1].
Polymer
There are several characteristics and types of polymers used for the electrospinning technique.
Melt vs Solution
The liquid used could be the polymer melt or the polymer solution. There are advantages and disadvantages to using either. With a polymer solution, a greater range of fiber diameters could be produced and the solution would be stable at room temperature. Micron sized fibers are easily produced with a polymer melt and involves no harsh solvents, unlike the polymer solution. Using polymer melts also allows for a simpler scale-up production but requires the melt to be maintained at higher temperatures [1].
Natural vs Synthetic
Many electrospun techniques used natural polymers for the use in tissue engineering because the polymers imitate the chemical and mechanical properties of the native biological environment of cells, such as the extracellular matrix. However, natural polymers are highly hydrophobic and viscous, making it difficult to manufacture. The fibers formed from the natural polymers also have weak mechanical properties [1].
Synthetic polymers, such as polylactic acid (PLA), polyglycolic acid (PGA, and polyethylene glycol (PEG), are highly used because the properties of the polymers are easier to optimize. However, the polymers do not have favorable biological properties that promote cell adhesion or cell proliferation [3].
To mediate this problem, two or more polymers can be co-spin together to create a co-polymer with characteristics of both polymers. Using co-polymers allows for better mechanical properties than the natural polymers and favorable biological responses compared to the synthetic polymers. These co-polymers can increase the hydrophilicity and affect degradation rate of the fiber mat. Incorporating a high biodegradable polymer with a low biodegradable polymer can produce a co-polymer with a degradation in between [14]. However, the process of using more than one polymer is more difficult. The polymers have to be cross-linked and there are limited choices in solvents that overlap with the synthetic and natural polymer [3].
Architectural Resemblance to the Native ECM
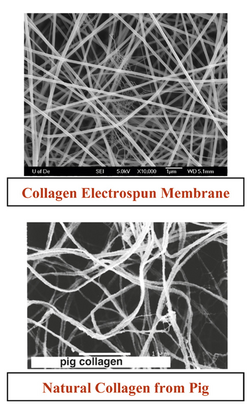
One of the main attractive characteristics of electrospinning is the ability to structurally imitate the extracellular matrix (ECM) [1]. Electrospinning creates a mat that has a high surface area to volume ratio and an interconnected three-dimensional porous network, crucial properties of the ECM [6]. Shown in Figure 3, a collagen electrospun mat show some resemblance to the natural collagen network found in pigs. In the ECM are several proteins including collagen, chitosan, gelatin, chitin, and hyaluronic acid. These are natural polymers, and independently, the polymers are difficult to electrospin on their own. The polymers also lack the physical properties of the ECM once electrospun. To overcome this obstacle hybrid polymers were created, Using PLGA with collagen and elastin improved the mechanical properties of the fiber mat compared to just the collagen and elastin alone [1]. By being able to incorporate the native proteins into the scaffold, along with the synthetic polymers, the synthetic ECM could potentially imitate the native ECM in its functions, such as promoting cell adhesion and migration or enabling diffusion of nutrients and wastes [6] Mimicking the native ECM allows for better cell adhesion and cell proliferation, making electrospinning a highly studied topic in tissue engineering [14].
Motivation
Wound Dressings
Perhaps one of the most researched applications is electrospun fibers used for wound dressings and wound healing (Figure 4). Non-fatal burn injuries are one of the major causes of infection and disease. Around 265,000 deaths each year are due to burn related injuries. Current wound dressings must be improved in order to better prevent infection and allow air diffusion through the bandage. A promising wound dressing alternative would be a bandage composed of electrospun fibers to treat burns and other related skin issues such as chronic ulcers. Electrospun fibers have many medical benefits including optimum porosity, and high surface-to-volume ratio. These properties, on a nanoscale basis, lead to enhanced natural skin healing, moisture retention and exudate removal. Therefore electrospun mats could provide wounds with an effective, protective barrier similar to the extracellular matrix (ECM). Previous work has shown that nanofiber mats increase cell proliferation, growth factors, and cellular ability to heal [2]. There is also research that suggest these wound dressings could not only aid in the healing process, but also serve as anti-microbial agents. In one study a solution of CA (0.5 and 5.0%) incorporated with chitosan/poly(ethylene oxide) (PEO) was successfully spun. These nanofibers demonstrated the ability to decrease activity rates of Escherichia coli and Pseudomonas aeruginosa. Thus electrospun fibers have the potential to combat bacterial infections [7].
Vascular Grafts
1.4 million patients per year need arterial prosthesis grafts. Current treatments include allografts, xenografts and synthetic grafts; however allografts are in limited supply, xenografts have a very short life span and synthetic grafts can pose poor integration problems. Composite electrospun scaffolds are being continuously researched as an alternative method for arterial prosthesis grafts. Different types of polymers have been researched for this purpose, including polyurethanes, collagen and chitosan just to name a few. These types of scaffolds have great potential in the vascular graft field; however more research needs to be conducted detailing cell seeding conditions as well as physical properties of the scaffold [8].
Bone Tissue Engineering
There is a great need to find bone tissue replacement alternatives instead of relying on autografts or allografts. Autografts are in limited supply and other foreign materials demonstrate poor integration with the patient's body. Electrospun materials have potential to be used as bone tissue scaffolds. Not only do electrospun fibers mimic the ECM, but these fibers can also be tuned and made into composite materials to optimize performance as bone scaffolds. In one study it was shown that a composite made from electrospun chitosan and hydroxyapatite nanoparticles could be crosslinked with genipin to create a very effective scaffold for non-weight bearing bone (cranial or maxillofacial). This composite material exhibited similar mechanical properties to bone tissue. Once seeded with osteoblast-like cells, the cells showed stellar proliferation, differentiation and maturation [9].
Nerve Injuries
Nerve injuries, particularly spinal cord injuries, can be debilitating and affect a person for the remainder of their life. Approximately 17,000 spinal cord injuries occur each year in the United States [10]. Depending on the gap between the damaged nerves, the nerve may be repaired. With a short gap, between 1 to 2 mm, the nerves can be repaired using sutures. Past 2 mm, the nerve damage begins to be irreparable and can no longer regenerate. For longer gaps, the current treatments have several disadvantages. In order to repair the damage, autologous nerve grafting is the most common treatment, but this requires the host endure multiple rounds of surgery and lose nerve function from the donor site [11]. Another challenge to repair nerve damage is how complex the nerve tissue is. After an accident or a surgery, scar tissue can form at the site of damage. The scar tissue can act as a barrier against the native nerve tissue in the body and prevent nerve regeneration [12].
Electrospun scaffolds are attractive in this field due to the ability to optimize fiber diameter, fiber alignment, and polymer characteristics. Alighned fibers have shown to promote neurite growth along the fibers, better than randomly aligned fibers. The electrospun scaffolds also can be optimized for a specific degradation rate. Spinal cord injuries can take up to several weeks or months to completely. The implanted scaffold should be able to last just as long in vivo. If the scaffold were to degrade prior the full recovery of the injury, the neurons may not grow correctly. A method of controlling the degradation for the polymers is to use co-polymers and slow degradation polymers such as PEG or PLA [12].
Nerve Guidance Conduits
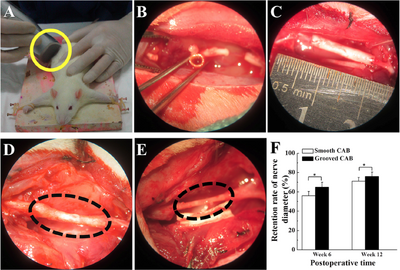
Researchers from Shanghai developed a nerve conduit from aligned nanofibers produced by electrospinning. The nerve guidance conduits were produced with a specific surface morphology, with grooves on the surface of the fiber, rather than a smooth surface. The conduits were made from cellulose acetate butyrate (CAB) as a model, due to its biocompatability [11].
Methods
The conduits were electrospun using a special collector consisting of two magnetic rods on each end of a glass rod. The glass rod was dipped into molten sugar to create a layer between the fiber and glass rod, for easy removal once the process is completed. As the fiber ejected from the needle tip, the collector rotated in order to create the desired cylindrical shape and fiber alignment for the conduit. Conduits with smooth surface fibers and grooved fibers were both produced for the experiment. The conduits were implanted into 24 mice, 12 for the smooth, 12 for the groove. Another group was used as a control, with an autograft implant. In all the rats, 13 mm segments of the sciatic nerve that runs in the thigh were all cut and removed from the rat and replaced by a 15 mm conduit. The autograft group of rats had the segment of the sciatic nerve cut, reversed, and implanted back into the leg. To analyze the nerve recovery, the gaits of the rats were observed throughout the experiment [11].
Results
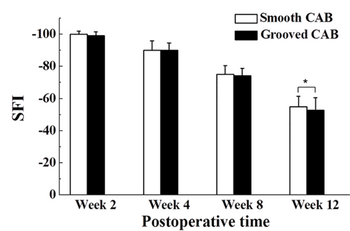
After 6 weeks, the regenerated nerves for the smooth conduit and the grooved conduit were found to be 56% and 65% of the nerve's original diameter, respectively. After 12 weeks, the regenerated nerve grew to 71% and 76%, respectively. SFI is an index that indicates nerve function. Normal nerve function is at 0, while complete nerve dysfunction is at 100. In the first weeks of the study, the conduit groups had no significant difference in nerve function. By the twelfth week, the grooved CAB conduit group demonstrated a significant difference in nerve regeneration compared to the smooth conduit group. The surface-grooved conduits also showed a higher density of neurofilaments in the regenerated sciatic nerve as well as a higher number of cells, compared to the smooth conduits.
To compare, the researchers used an autograft group. The results of autograft group demonstrated better improvement in nerve functionality than the two conduit groups. This is most likely due to the already present neurofilaments in the autografted nerve. The conduits did not have any proteins or factors to help nerve growth. The nerves were reconstructed at a slower and careful rate, due to the lack of neurofilaments.
Other Applications
Filtration
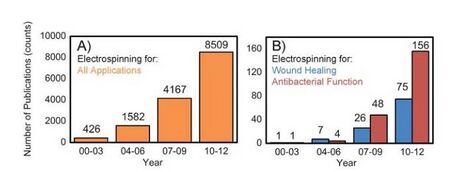
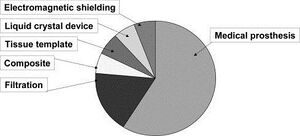
The diversity in electrospun material applications stems from the versatility and tunability of the system parameters as shown in Figure 7. For example, a large application for these fiber mats is filtration. Electrospun materials are beneficial in the filtration field not only because the polymer solution choice can be application defined, but also because these solutions can be impregnated with bacteria killing nanoparticles such as silver.[2] Filtration is actually the only electrospinning application that has reached the industry level. Freudenberg Nonwovens is a manufacturer for electrospun filter media that has been in business for over 20 years. Other electrospun filtration companies include Donaldson and Finetex [4].
Medical Prosthesis
Though filtration is the only application to reach industry level, judging by patents alone, medical prosthesis is the leading application for electrospinning [13]. This encapsulates a wide array of different grafts including skin, bone, and veins. Another application related to grafts are wound healing systems in which the nanofiber mat is used as a very powerful "band-aid" for large or chronic wounds. Research has also gone into drug delivery systems within these bandages. Drugs can be loaded in the polymer solution pre-spin or seeded into the fibers post spin [2]. The main reason for the large amount of research in the grafting and wound healing fields is due to the fact that the fibers are pours, non-woven, and have a large surface area to volume ratio. These qualities are all dominate in our own extracellular matrix, making these fibers a close replicate to our body's tissue.
Material Reinforcements
Another interesting application for these fibers are material reinforcements. Specifically, research has gone into using electrospun nanofibers along with carbon nanotubes to strengthen materials. One of the reasons this is a popular area of research is because of nanofibers outstanding mechanical properties [13].
Challenges
One of the biggest challenges with electrospinning is scale up. Since the process requires a small diameter needle, it would be difficult to scale this process up unless somehow hundreds of syringes were lined up and one large collector plate was used for fiber deposition. This method would increase cost greatly. Another issue with using electrospun fibers for tissue engineering is the pore size of the mats. While most human cells are upwards of 10 microns, poor size in an electrospun mat is typically less than 5 microns. This factor makes it difficult for cells to migrate and uniformly cover a surface. However to combat this obstacle certain materials (eg. pan-MMP) can be added to scaffolds to cleave sites so cells migrate.[13][4]
References
[1] Sill, T.J & von Recum, H. (2008) Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials, 29(13). 1989-2006.[1]
[2] Rieger, K. A., Birch, N. P., & Schiffman, J. D. (2013). Designing electrospun nanofiber mats to promote wound healing–a review. Journal of Materials Chemistry B, 1(36), 4531-4541. [2]
[3] Rim, N.G., Shin, C.S., & Shin, H. (2013) Current Approaches to Electrospun Nanofibers for Tissue Engineering. Biomedical Materials, 8(1). 1-14. [3]
[4] Teo, W. E., Inai, R., & Ramakrishna, S. (2016). Technological advances in electrospinning of nanofibers. Science and Technology of Advanced Materials.
[5] Li, S., Singh, J., Li, H., Banerjee, I. (2011). Electrospinning of Biomaterials. Biosensor Nanomaterials. John Wiley & Sons.
[6] Ratner, B., Hoffman, A., Schoen, F. , & Lemons, J. Biomaterials Science: An Introduction to Materials in Medicine. Third Edition. 332-339. Elsevier Science.
[7] Rieger, K. A., & Schiffman, J. D. (2014). Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly (ethylene oxide) nanofibers. Carbohydrate polymers, 113, 561-568. [4]
[8] Hasan, A., Memic, A., Annabi, N., Hossain, M., Paul, A., Dokmeci, M. R., ... & Khademhosseini, A. (2014). Electrospun scaffolds for tissue engineering of vascular grafts. Acta biomaterialia, 10(1), 11-25.
[9] Frohbergh, M. E., Katsman, A., Botta, G. P., Lazarovici, P., Schauer, C. L., Wegst, U. G., & Lelkes, P. I. (2012). Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials, 33(36), 9167-9178.
[10] National Spinal Cord Injury Statistical Center, Spinal Cord Injury (SCI) Facts and Figures at a Glance, 2016.[5]
[11] Huang, C., Ouyang, Y., Niu, H., He, N., Ke, Q., Jin, X., Li, D., Fang, J., Liu, W., Fan, C., & Lin, T. (2015) Nerve Guidance Conduits from Aligned Nanofibers: Improvement of Nerve Regeneration through Longitudinal Nanogrooves on a Fiber Surface. Applied Materials & Interfaces, 7. 7189-7196. [6]
[12] Schaub, N. (2016) Electrospun Fibers: A guiding scaffold for research and regeneration of the spinal cord. Neural Regeneration Research, 11(11). 1764-1765. [7]
[13] Huang, Z. M., Zhang, Y. Z., Kotaki, M., & Ramakrishna, S. (2003). A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites science and technology, 63(15), 2223-2253.
[14] Agarwal, S., Wendorff, J., Greiner, A. (2008). Use of electrospinning technique for biomedical applications. Polymer, 26(8). 5603-5621. [8]
