Drug Eluting Polymers and their Applications, by Eric Stowe
Background
Drug-Eluting Polymers
Polymers have increasingly and extensively been researched and used for a variety of biomedical, therapeutic, and tissue engineering applications. Polymeric hydrogels that have been engineered to respond to a range of different physical and chemical stimuli have been developed for drug delivery systems [6]. Polymer micelles and liposomes have been used for direct drug delivery and targeting of cancerous cells [7].
The above stated polymer drug applications are on the forefront of upcoming advanced research for a range of tissue engineering applications. However, the drug eluting polymer stent for treatment of blocked arteries and blood vessels has already been FDA approved and is used widely today [21]. The first drug eluting polymer stent that was launched was the Cypher stent in 2003, followed by the Taxus stent in 2004.
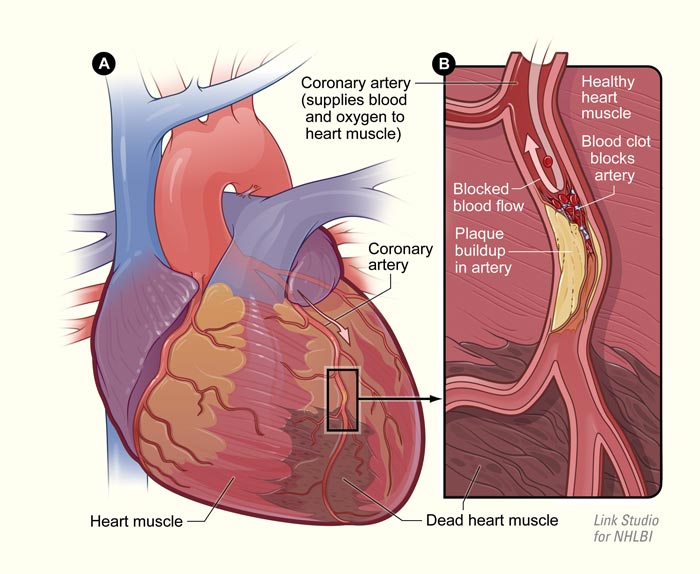
Coronary Artery Disease
Coronary artery disease, also known as heart disease, is the number one killer in America, affecting more than 13 million people [18]. Every year, nearly 610,000 people die of heart disease in the United States; 1 in every 4 deaths [19]. Heart disease is the condition that results due to a build up of plaque, cholesterol, glucose, or other substances in the arteries, which reduces and blocks blood flow to the heart. The arteries are naturally smooth and elastic endothelial cells, however, when plaque begins to build up, the arteries adopt a more narrow and rigid structure. With a lack of blood, nutrients, and oxygen flowing to the heart, the risks for heart attack and stroke elevate substantially.
The primary reason heart disease is so profound is due to the lack of nutrition and poor diets. Without proper nutrition and diet, fats and cholesterol-laden foods begin to deposit plaque in the blood vessel walls and arteries. Over time, the plaque begins to become too heavy on the artery walls, which causes them to narrow. Naturally, the plaque that is stuck to the vessel walls releases chemicals in an effort to heal, but this process actually provides negative impacts. The chemicals that are released make the artery walls sticky, which attracts other substances in the blood that pass through. Substances such as glucose and calcium quickly become attached to the walls, allowing them to become more narrow.
Motivation
There are numerous major and minor arteries throughout the human body, all which serve one function; to carry blood to or from the heart. Branched networks extend from each artery creating an intricate framework of blood delivery. As humans adopt poor diets and nutrition, along with the lack of exercise, arteries can become narrowed or blocked due to plaque buildup.
These blockages can reduce and slow blood flow to or from the heart, ultimately leading to heart attack, stroke, or death. The development of biocompatible platforms to unblock and widen clogged arteries has been studied since the mid 1900's, beginning with angioplasty, balloon angioplasty, metal-based stents, and polymeric drug eluting stents.
Through the use of these materials, arteries and blood vessels have been reopened to increase blood flow and supply nutrients to and from the heart, reducing the chances of heart attack and death in many people worldwide.
History
Coronary/Balloon Angioplasty
Percutaneous Transluminal Coronary Angioplasty, was developed in the mid 1960's and pioneered by Charles Dotter [2]. Previous arteriosclerotic obstruction, or artery thickening, treatments included therapy and nonsurgical techniques, which provided the patient only to live with the disease. Due to this, Dotter created the new coaxial angioplasty technique and reported his initial findings of its applications for arterial blocking in lower leg extremities of nine patients. All of these patients were rejected for definitive surgery and thus were scheduled for amputation. Dotter completed 15 procedures on 11 extremities and the success was immediately noted. Dotter's procedures showed six extremities that had increasingly improved blood pressure and circulation, and this subsequently eliminated the need for 4 amputations. He also observed three patients whose conditions did not change, two amputations that were not averted, and one amputation had the ability to be delayed by 3 months.
Following Dotter's success with this new coaxial angioplasty technique, its application was used extensively well into the late 1960's. In the early 1970, an improved balloon angioplasty technique was developed by Andreas Gruntzig and David Kumpe [3]. The balloon dilation application allowed for improved success in widening the blocked arteries and walls of patients. Gruntzig treated over 300 patients during the course of the 1970's and his balloon technique showed markedly improved success rates as compared to Dotters original coaxial technique. Gruntzig found that two year patency rates with the balloon dilation method was 86% as compared to the coaxial method's success of 64%. Furthermore, a lower complication rate was also observed; 8.5% with the balloon method as opposed to the 20% with the coaxial technique.
Bare-metal Stents (BMS)

In the late 1980's and early 1990's, new bare-metal stents were designed utilizing the balloon foundation that was built in earlier years. The metal stents primary effort was to reduce the complications of restenosis after the angioplasty procedure [16]. These metal stents were primarily expandable metal mesh cylinders that were mounted on the balloon and open out once the balloon is inflated. After inflation, the metal mesh stents line the artery walls and provide rigidity and structure, even after the balloon is deflated for removal.
This design was markedly improved compared to the balloon technique because the balloon technique only widened the artery during inflation and did not provide further structure after deflation. Once the balloon was deflated, the arteries and blood vessels were allowed to collapse and renarrow. This issue was improved by using metal stents because after deflation, the metal mesh provided a foundational support for continued opening of the artery.
One of the first uses for expandable metal stunts was done by Simonds et al. for treating bronchial obstruction in 1989 [4]. Using two case studies of patients who had blockages in their bronchia which caused pain and shortness of breath, metal stents were placed using balloon catheters lined with metal meshes. In the first case study, within a day of insertion of the metal stent into the bronchia, breathlessness had lessened substantially. Over the course of the next two years, the patient was monitored and showed no decrease in breathing capabilities. Chest radiographs also showed that there was no change in position of the metal stent. In Simonds second case study, another patient with shortness of breath was treated using a metal stent.
Remarkably, a reduction in breathlessness was exhibited upon recovery from the general anesthetic. Similarly no change in position of the stent was noted over the course of the next few months. This newly improved metal stent which built upon the foundations of the balloon catheter method was used well into the 1990's for heart disease.
Drug-Eluting Stents (DES)
Beginning in the early 2000's, drug eluting polymer stents were designed to address the problems of restenosis that persisted from the bare-metal stents [16]. Like the bare-metal stents, the drug-eluting polymer stents built upon the foundation of its predecessor.
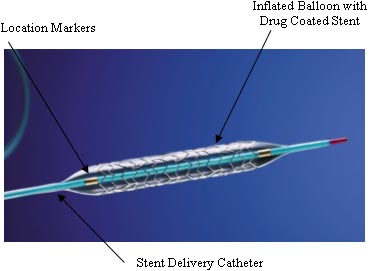
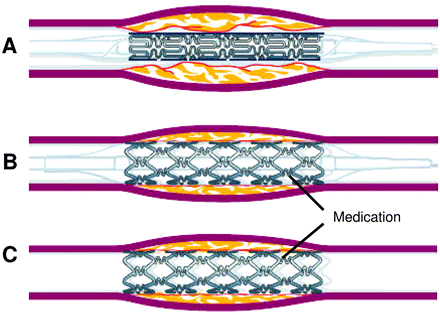
The drug eluting stents consist of a bare-metal stent coated with a drug encapsulated polymer which gradually releases the drug over time. These types of stents can be made in a variety of different ways which opens the opportunity for a vast amount of applications. The type of metal that is used for the mesh can be altered, and more importantly, the type of drug used and the release characteristics of the polymer can be varied [16].
Angioplasty Procedure
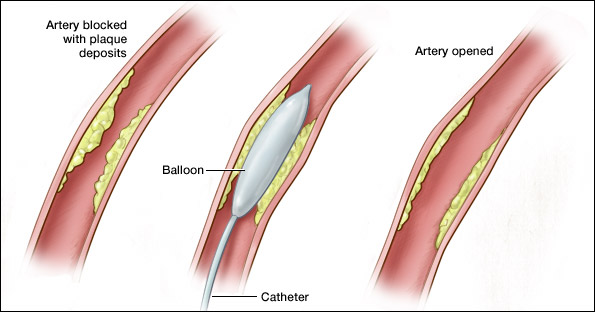
Angioplasty is the minimally invasive procedure through which a vascular surgeon inflates a small balloon inside an artery or blood vessel that has narrowed due to plaque buildup. The narrowing of the arteries or blood vessels restricts blood flow in the veins, and ultimately to the heart, which can cause heart disease and subsequently heart attack and stroke [18].
To alleviate the narrowing of arteries or blood vessels, vascular surgeons insert a small balloon, which upon inflation, forces the plaque against the arterial wall, allowing blood flow to become regulated. After the balloon has inflated and allowed the artery's blood flow to be re-established, it is deflated for subsequent removal. In newer techniques, the vascular surgeons insert a polymeric drug-eluting stent that contains a metal catheter foundation, and a balloon that is coated with a drug encapsulated polymer.
The metal catheter foundation aids the surgeons in placement and guidance to the diseased artery or blood vessel. Once at the diseased site, the drug encapsulated polymer coating delivers a steady dose of drug to the angioplasty trauma site. The drug is released to the newly opened artery over the course of six to nine months, which inhibits the arterial cells ability to proliferate and cause restenosis, the subsequent renarrowing of the artery due to the body's healing response [16]. Once the drug has been completely eluted, the polymeric stent remains intact for continued structural enhancement.
Challenges
Artery Wall Collapse and Restenosis
Restenosis occurs when the treated artery or becomes blocked again [22]. The typical timeframe for occurrence is usually within 6 months after the initial procedure. Restenosis was a primary issue when angioplasty first became a promising approach to fix clogged arteries.
Coronary/Balloon Angioplasty
Soon after the coaxial and balloon angioplasty became a break through in treating blocked arteries and blood vessels, several complications unfolded. In a small number of procedures and cases, it was found that the balloon inflation and deflation process weakened the artery wall so much that the artery wall collapsed [16]. The collapsing of the artery wall lead to the need for emergency bypass graft surgery. This artery wall collapse condition was for an extremely low quantity of cases, but the more common complication was restenosis. Within the first year of all procedures, restenosis was observed in 30 to 40 percent of patients [1].
Bare-Metal Stents
Similarly with the earlier methods for treatment of artery blockages, metal stents still presented the challenge of restenosis, although it was slightly improved. It was shown that in between 20 to 30 percent of treated cases, the artery was found to renarrow within 6 months of stent insertion [16]. This renarrowing led to the need for repeat procedures.
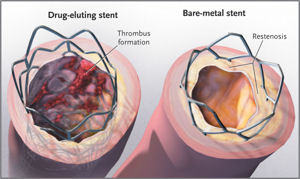
Drug-Eluting Polymer Stents
Over the years where polymer stents first began their use, widespread clinical data compared the use of drug eluting polymer stents with the bare-metal stent equivalent. This data overwhelmingly showed that the use of polymer stents significantly reduced the incidence of restenosis compared to metal stents; citing the restenosis incidence to under 10 percent [16].
Late Stent Thrombosis in Drug-Eluting Polymer Stents
Although the levels of restenosis had significantly decreased throughout the generations of stents that were developed, with the lowest levels in polymer stents, a new complication arose in the late 2000's from these drug eluting polymer stents. The new complication, late stent thrombosis, the formation of a blood clot, began to appear inside the stent more than a year after insertion [16].
Researchers have failed to pinpoint the exact cause of the late stent thrombosis, however, two hypothesis have been made. The first hypothesis is due to the antirestenotic drug which delays endothelialization of the artery and blood vessel walls. The second hypothesis is due to hypersensitivity reactions to the polymer which remains coating the metal stent once all the drug has been released [16].
Recent Studies
Second Generation Drug-Eluting Polymer Stents
In a real world study done by Sarno et al. in 2012, the researchers compared the use of second generation drug-eluting stents with the use of first generation drug-eluting stents and bare-metal stents [10].
First generation drug eluting stents were classified as: Cypher and Cypher Select, Taxus Express and Taxus Liberte, and Endeavor. Second generation drug eluting stents were classified as: Promus and Promus Element, XienceV and Xience Prime, and Endeavor Resolute.
After evaluation of 96,384 consecutive patients who underwent consecutive stent implantations over the course of November 2006 to October 2010, analysis of stent thrombosis, restenosis, and death in each case revealed promising results.
The rates of restenosis at 1 and 2 years after the procedure were found to be 6.3% and 7.4% in the bare-metal stent group, 4.0% and 5.8% in the first generation drug-eluting polymer stent group, and 2.8% and 3.9% in the second generation drug-eluting polymer stent group.
The rates for definite stent thrombosis at 1 and 2 years after the procedure were 1.2% and 1.4% in the bare-metal stent group, 0.9% and 1.3% in the first generation drug-eluting polymer stent group, and 0.5% and 0.6% in the second generation drug-eluting polymer stent group.
Further results indicated the mortality rates in comparison to each different type of stent that was used. The mortality rate for using bare-metal stents was found to be 6.8%, 3.4% when using the first generation polymer stents, and 1.9% when using the second generation polymer stents.
The primary overall finding from this study found that the use of second generation drug-eluting polymer stents was associated with a 38% lower risk of restenosis, and a 43% lower risk of definite stent thrombosis for up to 2 years as compared with the use of first generation polymer stents in a large real-world population.
Biodegradable Polymer Stents
In a 2014 study done by Wang et al., researchers compared the use of biodegradable polymer drug-eluting stents versus the use of second generation drug-eluting polymer stents for patients with coronary artery disease [11]. Biodegradable polymer stents were designed as the newer method compared to permanent polymer stents due to an effort to continually decrease the risks of late stent thrombosis associated with permanent polymer stents.
Permanent polymer stents remain intact even after the drug has been completely released, which has been hypothesized as one of the contributing factors for late stent thrombosis since the body has sensitivity reactions to foreign materials. The biodegradable polymer stents are designed such that the polymer biodegrades after all of the drug has been released, leaving behind the bare-metal mesh foundation.
The results from Wang's study found that 0.9% of patients developed stent thrombosis from the use of biodegradable drug-eluting polymer stents as compared with 0.8% of patients developing stent thrombosis from the use of second generation drug-eluting polymer stents. These results concluded that there was no statistical difference in the risk of definite or probable stent thrombosis between the two groups of stents tested.
Further results indicated no statistical difference in risks of all-cause mortality, and myocardial infarction between the two groups of stents used. In conclusion, the researchers found that biodegradable polymer drug-eluting stents have an equivalent clinical benefit to second generation drug-eluting polymer stent treatment with regard to stent thrombosis and myocardial infarction.
Bioresorbable Polymer Stents
The newest and most advanced method for percutaneous coronary intervention is through the use of bioresorbable vascular polymer scaffolds [12]. The first such device that is clinically being used worldwide is the ABSORB bioresorbable vasculary scaffold (BVS). The device uses a poly-L lactide polymer backbone, coated with a drug matrix consisting of a mixture of a second poly-D,L lactide polymer and the antiproliferative drug everolimus.
Both polymers undergo degradation and are completely bioresorbed in the human body within 24 months. The resultant soluble lactate monomers enter the Krebs cycle and are eliminated by the lungs and kidneys, eventually being converted to water and carbon dioxide.
It is hypothesized that the absence of residual polymer would potentially reduce the risk of very late stent thrombosis, which continues to be a problem for every polymer stent used to date. The overview provided by Sharma and Dzavik indicated that early studies that tested the bioresorbable vascular scaffold on simple coronary arteries reported no scaffold thrombosis out to 5 years [12].
Future Work
Through the advancement of several generations of coronary artery procedures, beginning with Dotter and Gruntzig's coaxial and balloon angioplasty, and moving through bare-metal stents and first and second generation drug-eluting polymer stents, and finishing with today's methods of biodegradable and bioresorbable polymer stents, the complications of restenosis and thrombosis have significantly been minimized.
However, the risks are not completely eliminated, even though the levels are below 1% with these new techniques. Future directions for using polymer drug-eluting stents for percutaneous coronary procedures would involve materials that completely eliminate the chances for restenosis and thrombosis over the patients life time. Current studies primarily look at the time period of 1-3 years after procedure for analysis of complications.
If such a device could be made that completely eliminated the risks of restenosis and thrombosis all while requiring only one procedure to be done, a permanent solution for heart disease could be made in the very near future.
References
[1] Baim DS. "Percutaneous Coronary Revascularization". In Dennis L. Kasper, Anthony S. Fauci, Dan L. Longo, Eugene Braunwald, Stephen L. Hauser, & J. Larry Jameson. Harrison's Principles of Internal Medicine (16th ed.) 2005. New York: McGraw-Hill. pp. 1459–1462.
[2] Dotter, C.T., Judkins M.P., "Transluminal Treatment of Arteriosclerotic Obstruction: Description of a New Technique and a Preliminary Report of its Applications." Circulation. 1964. American Heart Association. pp. 654-670.
[3] Gruntzig, A., Kumpe, D.A., "Technique of Percutaneous Transluminal Angioplasty with the Gruntzig Balloon Catheter." American Journal of Roentgenology. 1979. pp. 547-552.
[4] Simonds, A.K., Irving, J.D., Clark, S.W., "Use of Expandable Metal Stents in the Treatment of Bronchial Obstruction." Thorax 1989. pp. 680-681.
[5] Serruys, P.W., Jaegere, P.D., Kiemeneij, F.K., et al., "A Comparison of Balloon-Expandable Stent Implantation with Balloon Angioplasty in Patients with Coronary Artery Disease." The New England Journal of Medicine. 1994. pp. 489-495.
[6] Ahmed, E.M., "Hydrogel: Preparation, Characterization, and Applications: A Review." Journal of Advanced Research. 2015. pp. 105-121.
[7] Lian, T., Ho, R.J.Y., "Trends and Developments in Liposome Drug Delivery Systems." Journal of Pharmaceutical Sciences. 2001. pp. 667-670.
[8] Fattori, R., Piva, T., "Drug-Eluting Stents in Vascular Intervention." The Lancet. 2003. pp. 247-249.
[9] Jaffe, R., Strauss, B.H., "Late and Very Late Thrombosis of Drug-Eluting Stents." Journal of the American College of Cardiology. 2007. pp. 119-127.
[10] Sarno, G., Lagerqvist, B., Frobert, O., et al., "Lower Risk of Stent Thrombosis and Restenosis with Unrestricted use of 'New generation' Drug-Eluting Stents: A Report from the Nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR)." European Heart Journal. 2012. pp. 606-613.
[11] Wang, Y., Dong, P., Li, L., et al., "Biodegradable Polymer Drug-Eluting Stents Versus Second-Generation Drug-Eluting Stents for Patients With Coronary Artery Disease: An Update Meta-Analysis." Cardiovascular Drugs and Therapy. 2014. pp. 379-385.
[12] Sharma, V., Dzavik, V., "Bioresorbable Vascular Scaffolds: A New Revolution in Percutaneous Coronary Intervention?" Canadian Journal of Cardiology. 2015. pp. 247-249.
[13] Vascular and Interventional Radiology, "Peripheral Artery Disease." http://virchicago.com/peripheral-artery-disease/
[14] Odell, T., "Bare Metal Stents Show Promise for Restenosis Prevention." 2006. http://www.medgadget.com/2006/01/bare_metal_sten.html
[15] FitzGerald, S., "Making Sense of Stents." American College of Physicians Hospitalist. http://www.acphospitalist.org/archives/2007/05/best_practices.htm
[16] Biosensors International, "A Brief History of Drug-Eluting Stents." http://www.biosensors.com/intl/sites/default/files/pdfs/newsroom/des_history_review_6.7.11.pdf
[17] American Heart Association, "Drug-Eluting Stents." Circulation Journals. http://circ.ahajournals.org/content/115/17/e426/F1.expansion.html
[18] WedMD, "Coronary Artery Disease." http://www.webmd.com/heart-disease/guide/heart-disease-coronary-artery-disease
[19] Centers for Disease Control and Prevention, "Heart Disease Facts." http://www.cdc.gov/heartdisease/facts.htm
[20] NIH Medline Plus, "Heart Disease: Symptoms, Diagnosis, and Treatment." http://www.nlm.nih.gov/medlineplus/magazine/issues/winter09/articles/winter09pg25-27.html
[21] Food and Drug Administration, "FDA Approves Drug-Eluting Stent for Clogged Heart Arteries." http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116848.htm
[22] American Heart Association, "Restenosis: Repeat Narrowing of a Coronary Artery." Circulation. http://circ.ahajournals.org/content/105/22/2586.full
