Droplet Microfluidics: Synthesis of Janus Particles - Teresa Wang
Introduction
Droplet-based microfluidics as the new technology has enabled many experiments over the past 20 years. It has been used in many fields of research, such as chemistry, biology, physics and many engineering applications. Since the volume of each drop can be precisely controlled, it allows the size of droplets to be controlled by several operations including sorting, coalescence, splitting, mixing and trapping. From these manipulations, further analysis and chemical reactions could react under the desired conditions.
Droplet-based microfluidics isn't used to create just simple homogeneous droplets. It offers various paths to generate droplets through multiple microchannels. Complicated droplets, like the special designed droplets, can be formed by those complex fluids routes, such as concentric capillaries, T-junctions, and Y-junctions. Because of the special and unique technology of microfluidics, small volume, small size, low energy and low cost, it can be used in many applications at the intersection of several fields like biology chemistry and engineering. For example, DNA chips use microarray to determine the presence and amounts of proteins in the sample. Further more, microfluidics can be applied on molecule biology and cytology. It is not only important on biotechnology, but also creates a new way for synthesis of nanoparticles in chemistry and polymer science. One application that we talk about here is for multicompartmental droplets, or Janus particles. [1]
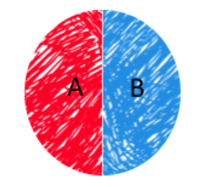
Janus was the ancient Roman god who had two faces. Therefore, Janus particles are a type of nanoparticles that contain two different surfaces on one particle. Those surfaces have distinct chemical and physical properties as shown in Fig. 1. Two surfaces can be different charges or hydrophobicity. For example, A is hydrophilic and B is hydrophobic. Due to these distinguishable surfaces, Janus particles can be applied in microrheology and bioimaging as microprobes. It has huge potential effect in physics, chemistry, biology and environmental science. For instance, Lahann's group reported a polymer-based Janus nanoparticles. [2] One hemisphere shows high binding affinity toward human endothelial cells, and the other hemisphere is resistance towards cell binding. [2]
Droplet Formation
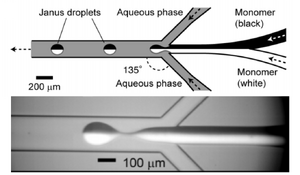
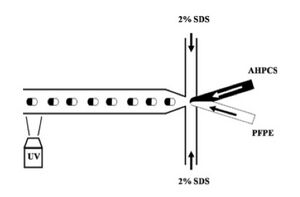
Microfluidics can be used for large-scale production of Janus particles. Synthesis of Janus particle takes advantage of microfluidics droplets, such as T-junctions, Y-junctions, or concentric capillaries. Co-flow systems can couple droplet formation with laminar flow to create the two-sides of the particle, and is one of the common mechanism used in Janus particles synthesis. In 2006, Torii from University Tokyo developed the process of hemispherical bicolored droplets as Fig. 2 showed. A Y-junction was first applied on a planar glass chip to create a two-phase organic flows in the steric stabilizer aqueous streams. In this system, two-phase flow using an oil-in-water emulsion was used to avoid convective transport between the two fluids. As shown in Fig. 2, the system takes advantage of laminar flow to create a Janus droplet by combining two separate organic streams. These Janus particles are then stabilized by the presence of a stabilizing polymer in the downstream area. Similar systems can also be used for creating the inorganic-organic Janus particles as in Fig. 3. [3] [4]
Based on the work of Toru[3], other research has been reported on a co-flow system with Y-junction. Kumacheva, from the University of Toronto, incorporated two different photoactive monomer chemistries to form two surfaces. Following droplet formation using the Y-channel device, UV light was used to polymerize the monomers and create stable Janus particles. Furthermore, the system can also be further extended to ternary particles as reported by Nie. This was achieved by flowing one monomer on the top and bottom and one different monomer in the center. In addition to changing the stabilizing solution or chemical initiator, the monomers can easily be replaced with other kinds of monomers enabling the formation of different functional surfaces for Janus particles. [3][5][6]
Synthesis of Janus Particles
In droplet-based mircrofluidics, the synthesis of Janus Particles can be easily applied through polymerization of both photoinduced and heat-introduced. A biphase co-flow system is most commonly used.
Janus droplets are easily formed by two monomers from Y-junction in the biphase co-flow system, and into the photoinitiator solution, follow by the UV-light irradiation. Professor Kumacheva and coworkers reported this process as Fig. 3 shown. For their experiments, they applied methacryloxypropyl dimethylsiloxane for M1, the mixture of pentaerythritol triacrylate (45 wt %), poly(ethylene glycol) diacrylate (45 wt %) and acrylic acid (5 wt %) for M2, and 2 wt% solution of sodium dodecylsulfate for W. In addition, the irradiation wavelength of UV-light is 200mm. [6]
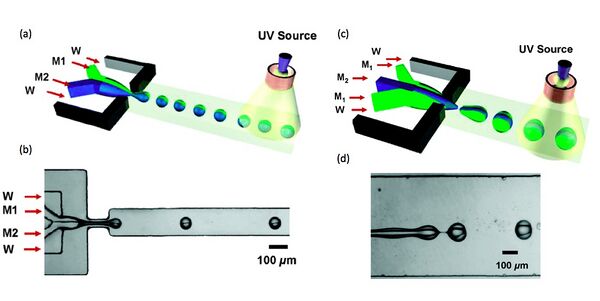
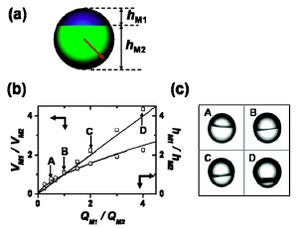
As Nie reported, the diameters of Janus droplets in Fig. 5 showing was about from 40 to 100 µm. To control the ratio of two surfaces of Janus droplets, they reported that the strategy is manipulating the volume fraction of the mixing monomers to adjust the flat interface between two separate phases. In Equation 1, Q is the flowrate of monomers, V is the volume, h is the height of each hemisphere, and R is the radius of the Janus droplet. The equation indicates that the ratio of flowrates M1 and M2 equals to the ratio of volumes, and also relates to the ratio of heights.
[math]\displaystyle{ \frac{Q_{M1}}{Q_{M2}}=\frac{V_{M1}}{V_{M2}}=\frac{{h_{M1}}^2(3R-h_{M1})}{{h_{M2}}^2(3R-h_{M2})} }[/math]
In Fig. 5b, the plot indicates that the variation in [math]\displaystyle{ \frac{V_{M1}}{V_{M2}} }[/math] with increasing ratio of [math]\displaystyle{ \frac{Q_{M1}}{Q_{M2}} }[/math]. Morphologies of Janus droplets are represented in Fig 5c with four Janus particles with volume ratios at 2/1, 1/1, 1/2 and 1/4 (A, B, C and D in Fig 5c). The top part of Janus particles is formed by M1. [6]
Furthermore, the biphase dispersed flow not only produces Janus droplets, but also the ternary structure droplets can be created by just adding M2 in the center, and two M1 flows on two sides. It means introducing three parallel co-flows at the same time, and the droplets coming out will contain two M1 monomers on the top and bottom, one M2 monomer in the middle. In addition, M1 can only be the intermediate channels and M2 be the middle channel. If M1 is in the central inlet, top and bottom M2 will merge and engulf M1. The reason for this is relative affinity of each monomer to the surface of the device. And M1 has low affinity keep the ternary structure stable.[6]
References
1. Shang, L.; Cheng, Y.; Zhao, Y. “Emerging Droplet Microfluidics.” Chem. Rev. May 24 2017, 117, 7964-8040, https://doi.org/10.1021/acs.chemrev.6b00848
2. Yoshida, M.; Roh, Kyung-Ho.; Mandal, S.; Bhaskar, S.; Lim, D.; Nandivada, H.; Deng, X.; Lahann, J. "Structurally Controlled Bio-hybrid Materials Based on Unidirectional Association of Anisotropic Microparticles with Human Endothelial Cells". Advanced Materials. 2009, 21 (48), pp 4920–4925. https://doi.org/10.1002/adma.200901971
3. Nisisako, T.; Torii, T.; Takahashi, T.; Takizawa, Y. “Synthesis of monodisperse bicolored Janus particles with electrical anisotropy using a microfluidic co-flow system.” Adv. Mater. 2006, 18, 1152-1156, https://doi.org/10.1002/adma.200502431
4. Naveen, P.; Jayakumar, P.; Chang-hyung, C.; Chang-Soo, L.; Dong-pyo, K. "Generation of monodisperse inorganic-organic Janus microspheres in a microfluidic device" Adv. Funct. Mater., 2009, , 1656-1662, https://doi.org/10.1002/adfm.200801181
5. Prasad, N.; Perumal, J.; Choi, C. H.; Lee, C. S.; Kim, D. P. “Generation of monodisperse inorganic-organic Janus microspheres in a microfluidic device.” Adv. Funct. Mater., 2009, 19, 1656-1662, https://doi.org/10.3390/mi8010022
6. Nie, Z.; Li, W.; Seo, M.; Xu, S.; Kumacheva, E. “Janus and Ternary Particles Generated by Microfluidic Synthesis: Design, Synthesis, and Self-Assembly.”J. Am. Chem. Soc., 2006, 128 (29), https://doi.org/10.1021/ja060882n
7. Lone, S.; Cheong, I. W. “Fabrication of polymeric Janus particles by droplet microfluidics.” RSC Adv., 2014, 4, 13322-13333, https://doi.org/10.1039/C4RA00158C
8. Shah, R. K.; Kim, J. W.; Weitz, D. A. “Janus Supraparticles by induced phase separation of nanoparticles in droplets.” Adv. Mater. 2009, 21, 1949-1953, https://doi.org/10.1002/adma.200803115
