Dahlquist:RNA-seq Protocol
The protocol below was adapted from the "SMART protocol for RNA-seq - revised June 3, 2016" by Arthur Hunt in the Department of Plant and Soil Sciences at the University of Kentucky and used at the GCAT-SEEK Workshop held at Cal-State Los Angeles on June 27 - July 1, 2016. Further annotations were made by Kam D. Dahlquist.
Reagents, Supplies, and Equipment
- Reagent List for GCAT-SEEK 2016 Workshops by Arthur Hunt
- Excel version of reagent list with calculator
- New England Biolabs NEBNext® Poly(A) mRNA Magnetic Isolation Module, catalog number E7490L.
- Invitrogen MagnaRack, catalog #CS15000
- SMARTSCRIBE, 100 units/µL, Takara Clontech, catalog #639537
- RNase Inhibitor, Human Placenta 40,000 units/mL, NEB catalog #M0307L
- Magbio Genomics, High Prep PCR Clean Up System (5 mL), catalog # AC-60005
- Phire Hot Start II DNA Polymerase (Thermo Fisher catalog #F-122S)
Equipment
- Invitrogen MagnaRack, catalog #CS15000
- thermocycler set to 65°C
- thermocycler set to 80°C
- thermocycler set to 95°C
- thermocycler set to 42°C
Notes
- It is reasonable to do 12 samples at a time with this protocol. The magnetic rack holds 12 tubes.
- All of the procedures, except for the PCR is performed in 0.5 mL tubes because thermocyclers are used for the incubations.
1. Poly(A) Enrichment
This protocol uses the New England Biolabs NEBNext® Poly(A) mRNA Magnetic Isolation Module, catalog number E7490L.
- Aliquot 10 μL of oligo(dT) beads into 0.5 mL microfuge tubes.
- Make sure the stock solution is evenly mixed before aliquotting.
- NOTE – this amount of the NEB beads suffices for quantities of total RNA below 10 µg. We have not explored larger quantities of RNA, as this seems exorbitant. Also, we have used as little as 100 ng of total RNA with good results. Lower quantities should work as well, but one should keep in mind that non-specific binding of RNA to beads may reduce the enrichment of poly(A) RNA. Also, it is probable that the starting amounts of poly(A) RNA may fall below the affinities of RT for template DNA if one starts with less than 100 ng of total RNA. This needs to be accounted for in the following steps (specifically, step 2), possibly by extending RT times and perhaps periodically adding more enzyme.
- Wash the beads twice with 100µL of RNA Binding Buffer (included with kit, 20mM Tris-HCl pH 7.5, 1.0M LiCl and 2mM EDTA), and remove the supernatant.
- Flicking or vortexing to mix the beads introduces air bubbles; gently pipetting up and down ~3 times seems to work well for mixing.
- Open the lids of the tubes before putting in the magnetic stand. The open cap fits in the notch and helps hold the tube in place. For additional stability, put your finger near the top of the tube to press it towards the back of the stand. The "pellet" of the beads will be to the back.
- Resuspend the beads in 50 µl of RNA Binding Buffer. (Pipet up and down 3X to mix).
- Bring RNA to 50 µL with RNAse-free water. Heat to 65°C in a thermocycler, cool on ice, then add to the washed beads. Incubate at room temperature for 5 min.
- Note that the RNA can be aliquotted and water added ahead of time and stored at -80°C.
- Collect beads using the magnetic stand, discard supernatant.
- Wash the beads twice with 100 µL of Washing Buffer (from kit, 10mM Tris-HCl PH 7.5, 0.15M LiCl, 1mM EDTA).
- Remove the supernatant from the beads, add 15 µL of 10mM Tris-HCl (from the kit) and heat the beads at 80°C for 3 minutes in a thermocycler to elute mRNA.
- Remove beads with the magnetic stand, save the supernatant in a fresh 0.5 mL tube.
- Note that it is important to not let the tubes incubate for longer than 3 minutes at 80°C because of the danger of hydrolysis of the RNA when incubated at high temperature for extended periods. It is also important to separate the eluate from the beads immediately after the incubation. Thus, if you are doing a lot of samples, stagger the times you add them to the thermocycler for incubation by 15-20 seconds. (I did 15 seconds apart at the workshop and ended up being a cumulative 20 seconds behind after I did all 12 of my samples, so 20 seconds would probably be better — Kam D. Dahlquist 23:37, 28 June 2016 (EDT)
- NOTE: it may be advisable to repeat this, to remove as much rRNA as possible. However, this is still an open issue, since organellar and stable RNAs are known to be polyadenylated, which means repeated rounds of poly(A) enrichment yielding reduced returns.
- Also, since we use NEB oligo-dT beads, it should be OK to follow NEB’s protocol for poly(A) enrichment instead of the one I described in the preceding. NEB and other companies sells kits as well as just the beads, and some may prefer to use the ready-made solutions and protocol.
2. RNA fragmentation, cDNA synthesis, and clean-up
- To the 14.5 µL RNA collected in the previous step, add 1 µL (=100 pmol) RT primer + 5 µL 5X 1st strand buffer.
- Note that each sample gets a different primer with a different bar code sequence so that the samples can be mixed for sequencing and then bioinformatically separated again later for analysis. It is critical to write down the bar code/sample correspondence.
- Heat to 95°C for 2 minutes in a thermocycler, chill on ice.
- NOTE – the high temperature in the RT buffer is a suitable and efficient proxy for stand-alone RNA fragmentation kits or systems. By folding fragmentation directly into the RT reactions, the process is streamlined.
- Immediately (while cold!) add 5 µL of Master Mix containing:
- Per sample:
- 2.5 µL 10 mM dNTPs
- 1 µL 20 mM DTT from SMARTSCRIBE
- 0.5 µL RNase Inhibitor, Human Placenta 40,000 units/mL, NEB catalog #M0307L
- 1 µL SMARTSCRIBE, 100 units/µL, Takara Clontech, catalog #639537
- Mix/flash spin and incubate for 120 min at 42°C in a thermocycler.
- Add 1 µL of the strand-switching primer (SMART7.5) and an additional 1 µL of SMARTSCRIBE, mix/flash spin and incubate for an additional 120 min at 42°C in a thermocycler.
- Heat samples to 70°C for 5 min.
- This is a convenient stopping place. Samples can be stored at -20°C.
- Remove 10 µL of the reaction to a new 500 µL tube and add 15 µL of 10 mM Tris HCl pH7.5 (from NEB kit above). Cap and save the remaining cDNA reaction (this is kept in reserve in case something bad happens in the subsequent parts of the procedure).
- To the diluted cDNA, add 16.25 µL SPRI beads that have been completely mixed and brought to room temperature. Incubate for 8 min at room temperature.
- Beads are from Magbio Genomics, High Prep PCR Clean Up System (5 mL), catalog # AC-60005
- This ratio of beads binds to large cDNAs. Products smaller than 300 bp will be washed away.
- Separate beads using the magnet stand (this may take a minute or two, because of the viscosity of the bead solution), remove and discard the supernatant.
- While the tube is on the magnet stand, add 100 µL fresh 80% ethanol. After 5-30 seconds (it doesn't matter, the point here is to wash the pellet; no need to resuspend the beads), remove and discard the supernatant. Repeat the wash.
- The 80% ethanol must be diluted fresh the same day the procedure is performed because ethanol is hygroscopic. Washing with lower percentages of ethanol may wash away some of the cDNA sample you want to recover.
- Air dry the washed beads for 10 min at room temperature.
- Add 100 µL 10 mM Tris HCl, pH 7.5. Mix, and then collect the beads with the magnetic stand. Remove the supernatant to a new 500 µL thin-walled microcentrifuge tube.
- Add 55 µL SPRI beads that have been completely mixed and brought to room temperature. Incubate for 8 min at room temperature.
- This ratio of beads binds to very large cDNAs, which will be gotten rid of because we are discarding the beads and keeping the supernatant below.
- Separate beads using the magnet stand (this may take a minute or two, because of the viscosity of the bead solution), remove and save the supernatant to a new 500 µL tube.
- Add 10 µL SPRI beads to the reserved supernatant from step m. Incubate for 10 min at room temperature.
- This ratio of beads binds cDNAs in the range of 400-700 bp. Smaller fragments will be washed away.
- Separate beads using the magnet stand, remove and discard the supernatant.
- While the tube is on the magnet stand, add 100 µL fresh 80% ethanol. After 5-30 seconds (it doesn't matter, the point here is to wash the pellet; no need to resuspend the beads), remove and discard the supernatant. Repeat the wash.
- Note that at this point the amount of beads is small. Care should be taken to avoid disturbing the pellet or otherwise losing the beads.
- Air dry the washed beads for 10 min at room temperature.
- Add 25 µL 10 mM Tris HCl, pH 7.5. Mix, and then collect the beads with the magnetic stand. Remove the supernatant to a new 500 µL thin-walled microcentrifuge tube. This is the final library to be used for PCR amplifications.
- The two-step process for RT and strand-switching was arrived at empirically in my lab, with guidance from some prior research publications. For us, the goal has been to develop procedures that can be used with sub-microgram quantities of RNA. I reasoned that a limiting factor would be the performance of RT, which is likely working below the Km’s for template. The easiest way to address this is to extend incubation times, hence the long reaction times.
- Also, while we have incubated the reverse transcription reactions at 42°C routinely for our libraries, it is probably OK to use 37°C as well. Lowering the temperature may reduce possible GC biases in terms of where cDNA synthesis is initiated on the template. We have not looked into this experimentally, but this is something to keep in mind.
- The elaborate SPRI bead process used here has been empirically determined to yield libraries with a size range of 300-600 bp, with most of the library in the 500 bp range. This process can be fine-tuned, and probably needs to be checked occasionally to identify lot-to-lot variability in the SPRI beads.
3. Checking Library Quality with PCR Amplification
Set up Phire Hot Start II DNA Polymerase (Thermo Fisher catalog #F-122S) PCR reactions using the PE-PCR1 and PE-PCR2 primers. The total reaction volumes should be 25 µL, and 1 µL of the library should be used as a template. Make a master mix with all the components of the reaction, except the template, and add 24 µL of it to 0.2 mL PCR tubes containing the template.
Component Volume for 1 rxn template cDNA 1 µL 5X PHIRE reaction buffer 5 µL (has 7.5 mM MgCl2 in it) 2.5 mM dNTP mix 2.5 µL 100 µM PE-PCR1 primer 0.1 µL 100 µM PE-PCR2 primer 0.1 µL water 15 µL Phire enzyme 0.5 µL TOTAL volume 25 µL
The thermocycler program is shown below. The annealing temp is 60°C (for 15 sec), and the extension time is 60 sec. (In principle, the extension time can be decreased, perhaps to as little as 30 sec; I use 60 sec to make sure every amplicon is completed, so that no odd PCR strand-switching artifacts arise later due to the buildup of truncated products.)
30 sec 98°C initial denaturation step 15 sec 98°C denaturation 15 sec 60°C annealing 60 sec 72°C extension (back to denaturation) 5 min 72°C final extension step Hold at room temperature
- As far as cycle # is concerned, a bit of range finding is usually needed. With 1 µg of total Arabidopsis RNA, 15 cycles usually works fine. Some members of my lab have done as few as 9 cycles here. At the other end of the spectrum, we have made libraries after as many as 24 cycles of amplification. As a rule of thumb, we try to avoid libraries involving more than 18 or 21 cycles, but sometimes this is unavoidable. Cycle numbers greater than 18 should not be needed if starting with 100 ng or more of total RNA.
- To optimize this for the cDNA libraries that were just generated, pick three cycles in the range of 9-12-15-18-21-24 to test and run three sets of PCR reactions in parallel. For example, you could test 15-18-21.
- At the GCAT-SEEK Workshop, we did two sets of paralell reactions with 21 and 24 cycles.
- Regardless of these considerations, the goal is a smear between 300 and 600 bp; if successful, the PCR amplification is then performed again using the lowest cycle number that gives sufficient product. The PCR products are then purified using an abbreviated SPRI bead protocol.
- We use Phire routinely for this step, because of a combination of the excellent and reliable performance of the enzyme and its relatively low cost (compared with some other hot-start enzymes). On occasion, we have used other Taq polymerases successfully, including “home-made” enzyme. The choice to include Phire here is intended to provide some measure of consistency; different enzymes can probably be used, but amplification conditions need to be optimized.
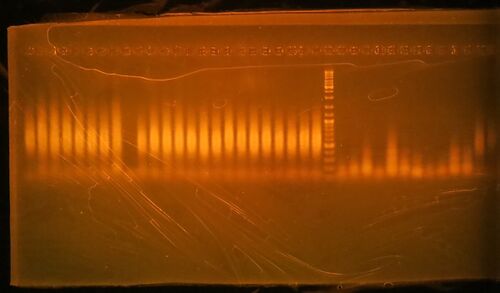
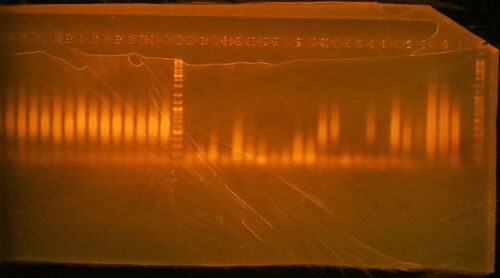
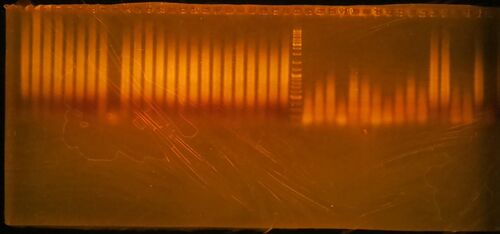
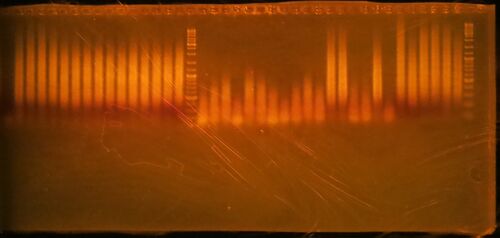
- Run 20 µL of the 25 µL PCR reaction on a 1.5% agarose/1X TBE gel stained with ethidium bromide. Gels generated at the workshop are shown. The marker is GeneRuler DNA ladder mix, ThermoFisher catalog #SM0331
- Click here for the key to the lanes on the gels.
Final library amplification and purification
- Repeat the PCR amplification of Step 3 with the appropriate number of cycles that you have determined empirically. This time, you won't run the products on the gel, but purify them using the SPRI beads.
- To each 25 µL PCR reaction, add 16.25 µL SPRI beads that have been completely mixed and brought to room temperature. Incubate for 8 min at room temperature.
- Separate beads using the magnet stand (this may take a minute or two, because of the viscosity of the bead solution), remove and discard the supernatant.
- While the tube is on the magnet stand, add 100 µL fresh 80% ethanol. After 5-30 seconds (it doesn't matter, the point here is to wash the pellet; no need to resuspend the beads), remove and discard the supernatant. Repeat the wash.
- Air dry the washed beads for 10 min at room temperature.
- Add 25 µL 10 mM Tris HCl, pH 7.5. Mix, and then collect the beads with the magnetic stand. Remove the supernatant to a new 500 µL thin-walled microcentrifuge tube. This is the final library that will be submitted for Bioanalyzer analysis and sequencing.
- Perhaps the most unsettling aspect of this is the fact that, often, one cannot know about library quality until the sequencing results are returned – this means one runs the risk of spending money on poor libraries. In my lab’s experience, size-selection and re-amplification is a good quality-control check for libraries. The most persistent problem with RNA-seq libraries is the presence of short amplification products that arise due to the direct copying of the strand-switching primer by RT, using the RT primer as a primer for reverse transcription. These can easily over-amplify and give smears that can be mistaken for good libraries. If these artifacts pass through the size selection and are re-amplified, the results are not a narrow band corresponding to the region expected based on the size selection process, but a long smear extending from 100 bp or so through the 1000+ bp size range. Some examples are shown at the end of this document.
Final Quality Check, Quantitation, and Submission for Sequencing
- After the workshop, Arthur takes the final amplified libraries back to his lab and runs them through the Agilent BioAnalyzer to judge quality and uses the Qubit for fluorometric quantitation of the cDNA libraries.
- Alternately, the PCR products can be run on an ethidium-bromide stained agarose gel as before to check the quality.
- The NanoDrop spectrophotometer (or NanoPhotometer) is not an adequate substitute for the Qubit. cDNA amounts are too small to be accurately quantitated in that way.
- Equal amounts of the libraries that will be run in a single lane will be mixed together. This is usually about 10 ng, but will be limited by the library with the least cDNA in it.
- Samples will be sent to the University of Delaware Sequencing & Genotyping Center, which has an Illumina HiSeq 2500.
- For outside investigators 1 x 76 cycles are $1,700 per lane.
- They will not do a run unless all 8 lanes of High-Output flow cell are filled up.
Useful Links
- How do SPRI beads work? by James@cancer.
- watermelon_pValue_example
- 2016 NGS Field Guide
- Alternate to magnetic rack, the Sunrise Scientific PickPen Magnetic Tool
- Workshop PBWorks page (private)
- GCAT-SEEK Web site
- p-hacking: Hack your way to scientific glory
- Small World Initiative
- lemon tree
- MultiQC: Aggregate results from bioinformatics analyses across many samples into a single report
