Crystal Violet Staining
Introduction
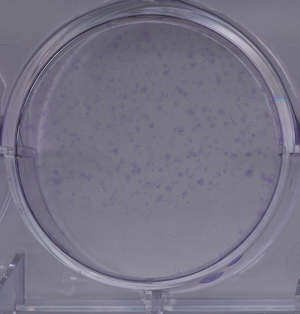
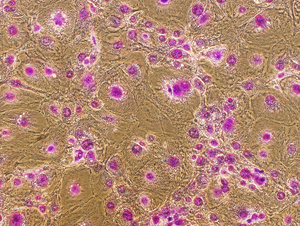
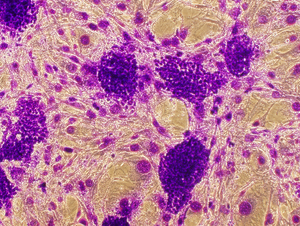
Crystal Violet staining stains nuclei a deep purple color, aiding in their visualization. It can also be used to visualize colonies of cells. The entire staining protocol takes less than an hour.
Staining Adherent Cells with Crystal Violet
- Place cells on ice and wash 2X with cold PBS (keep in refrigerator).
- Fix for 10 minutes with ice-cold 100% methanol (keep in freezer).
- Aspirate methanol from plates. If you want to stop here, cover the cells with 50% glycerol in PBS, wrap the plate with parafilm or plastic wrap, and keep in the refrigerator. They will be find for at least a few weeks.
- Move the cells off ice to room temperature and add cover them with 0.5% crystal violet solution in 25% methanol. Incubate for 10 minutes. Remove the crystal violet---it can be reused or disposed of as toxic waste.
- Wash the cells in water several times, until the dye stops coming off. A quick way to do this is to submerge the entire dish in a container full of water, then dump the water out and refill it a few times.
- Allow the cells to dry at room temperature (maybe overnight). They can be stored for a long time at room temperature.
Warnings
- Always pipette on the sides of the dish to avoid detaching cells
- Crystal violet is toxic and should be handled carefully.
0.5% Crystal Violet Solution (100 ml)
- 500 mg Crystal Violet
- 25 ml Methanol
- 75 ml Water
- Store at room temperature