Critical micelle concentration (CMC)
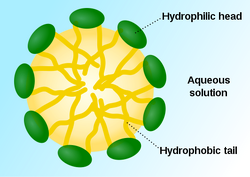
Critical micelle concentration (CMC) is defined as the concentration of detergents above which micelles are spontaneously formed. The CMC is important in biology because at concentrations above it the detergents form complexes with lipophilic proteins. Below this borderline, detergents merely partition into membranes without solubilising membrane proteins.
| Detergent | CMC (%w/v) | CMC (mM) | MW | Type |
|---|---|---|---|---|
| BRIJ 35 | 0.11 | 0.09[1] | 1200 | non-ionic |
| NP-40 | ~0.02[2] | 0.05-0.3[1] | ~650 | non-ionic |
| Saponin | ~0.1[3] | mixture | non-ionic | |
| Triton X-100 | ~0.02[2] | 0.2-0.9[1] | ~650 | non-ionic |
| Tween 20 | ~0.0074 | ~0.06[1] | ~1228 | non-ionic |
| SDS | 0.23[2] | 7-10[1b] | 288.5 | ionic |
| CHAPS | 0.49[2] | 6-10[1b] | 615 | zwitterionic |
Note: The molecular weights for some detergents are average values. Triton X-100, for example, can range between 600 and over 650 MW depending on synthesis. The exact molecular weight influences the CMC.
Note: CMCs can vary with ionic strength and temperature. For ionic detergents the CMC is reduced by increasing the ionic strength of the solution, but is relatively unaffected by temperature. For non-ionic detergents the CMC is relatively unaffected by ionic strength, but increases significantly with higher temperature. Ranges refer to a 20-25ºC temperature range.
Note: Saponin is a class of amphiphilic, natural metabolites with detergent properties often extracted from plants. CMC may vary.
Sources
- [1] newer (year?), abbreviated detergent guide, Calbiochem
- [1b] 2001 detergent guide, Calbiochem
- [2] Commonly used detergents, Frost lecture, UFL
- [3] Urum et al 2004, incl. data on saponin