Checking the RNA integrity on gel electrophoresis
From OpenWetWare
Jump to navigationJump to search
Preparation of the gel
- Prepare a 1.5-2% gel electrophoresis.
- For example, for a small gel, add 0.53 g agarose and 35 ml TBE/TAE to an Erlenmeyer flask.
- Microwave for 30-45 seconds, stop and swirl, and then continue towards a boil until the agarose is completely dissolved.
- Let the agarose solution cool down and add 3 µl of 'redsafe' (the amount depends on the 'redsafe' company).
- Pour the agarose into a gel tray with the well comb in place.
- Use the smallest well comb.
- For a larger gel, use 1.2 g agarose, 65 ml TBE/TAE, and 5 µl 'redsafe'.
Prepare samples
- Loading buffer (x6) - 1 ul
- From each sample, load at least 100 ng (500 ng to 1 ug is optimal).
- Add DDW up to a 6 ul final volume.
Running the gel
- Once the gel has solidified, place the agarose gel into the gel box (electrophoresis unit).
- Fill the gel box with 1xTAE (or TBE) until the gel is covered.
- Carefully load a molecular weight ladder (100 bp) into the first lane of the gel and carefully load your samples into the additional wells of the gel.
- Run the gel at 120V for 20-30 minutes.
Expected results
- RNA occupies 80% or more, followed by tRNA with 14% and ending with mRNA at about 2.5%.
- Therefore, when running RNA on the gel, you will see mainly the rRNA.
rRNA as a quality reference
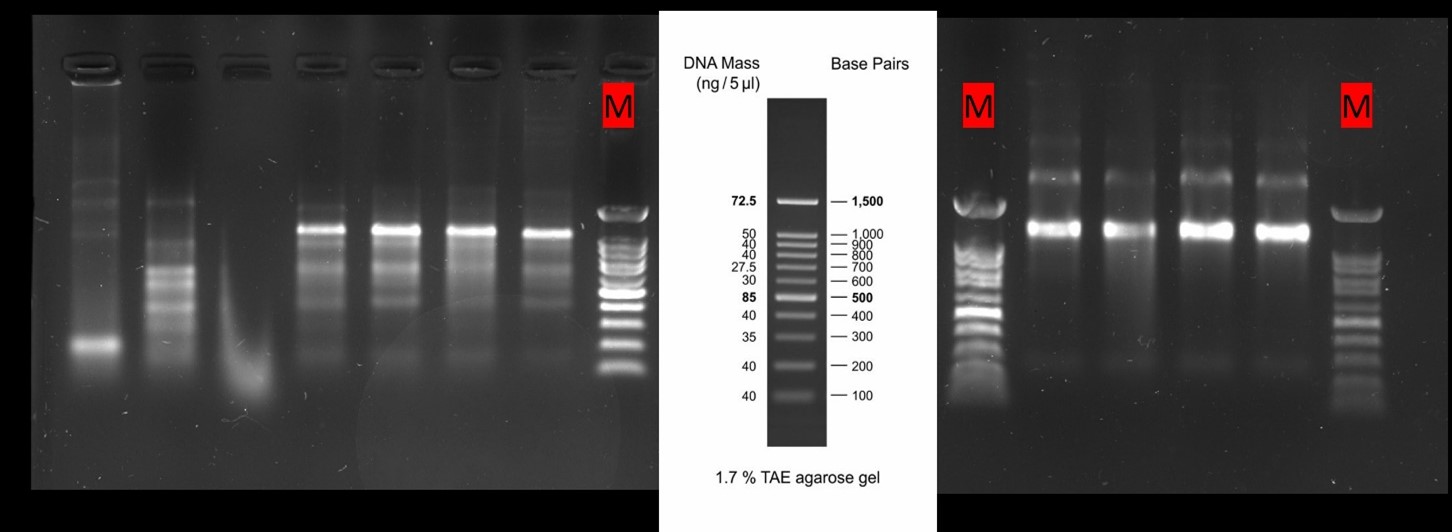
- Researchers expect to find in their RNA gels two bands, each corresponding to one subunit of rRNA (28S and 18S bands).
- Then, the hypothesis is, if rRNA subunits look okay and are not smeared, mRNA will also be intact.
- On the right, high-quality RNA integrity extracted from the BSF, and on the left, bad-quality (smeared) RNA extracted from the BSF.