Ceramic Biomaterials, by Jon Velez
Background
What are ceramics?
Ceramics are defined as “inorganic, non-metallic materials”. [3] They are hard and brittle, great strength and stiffness, wear and corrosion resistance, and low density; ceramics work great with compressive forces, and are electrical and thermal insulators. Conversely, ceramics are also at risk of having cracks or other defects, and have been used less extensively than metals and polymers. They are used in dentistry, orthopedics, and as medical sensors. [2]
Why bioceramics?
Bioceramics are important in the biomedical field due to their chemical similarity to bone; and are ideal for surgical implants due to their thermal and chemical inertness, and have high strength, wear resistance, and durability. Ceramic biomaterials also stimulate bone growth and have low friction coefficients. They do not create strong biologically relevant interfaces with bones, but they do promote strong adhesions to bones.[3] The main applications of ceramic biomaterials include:
-Joint/tissue replacement
-Metal coating to improve biocompatibility
-Resorbable lattice to provide a temporary structure that is eventually replaced by the body’s tissues
Bioceramics are not exclusively resorbable, they can also be bioactive, biodegradable, and soluble; and are also available as composites with a polymer component and microspheres.[1]
History
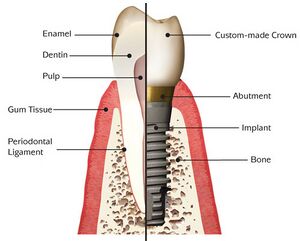
1800s – porcelain used as dental crowns[1]
1892 – common plaster of Paris to repair bone defects[1][3]
1920 – tricalcium phosphate successfully used[1]
1960’s – Zirconia first used as an implant materials[1]
1963 – Cerosium developed as a ceramic bone substitute. Cerosium is a porous aluminate ceramic impregnated with epoxy resin[3]
1965 – Alumina Patented[1]
1970 – First time Alumina was used as an implant[1]
1971 – first bioactive glass invented[1]
1980’s – first use of bioceramics in human surgeries, calcium phosphate and hydroxyapatite coated implants become commercially available[1]
1998 – TH-Zirconia implants become available.[3]
Types of Bioceramics
-Bioactive: Bioglass/glass ceramic
-Bioresorbable: Calcium phosphate
-Bioinert: Alumina,zirconia,carbon
Bioactive Ceramics
Bioactive materials are those that chemically bond with bone or tissue of the hosts.[2] The most important applications of bioactive bioceramics has been metal coatings to provide bone-implant interfacing, this lowers the risk of rejection and transmission of diseases.[3]
Bioglass and Glass ceramics
Glass is made of Silica, Calcium oxide, and Sodium oxide (SiO2-Na2O-CaO), and bioglasses used for implantation are based on glass with at least 65 weight percent Silica. Bioglasses have high mechanical strength and are bioinert, but are also brittle and have poor tensile properties. [1][2] [1] They are normally used in bone plating, dental implants, spinal fusions, and more. [2] In 1971 the first bioglass, 45S5 bioglass, was created. It was unusually weak with a composition of 45% Silica, 24.5% Calcium oxide, and 24.5% Sodium oxide. The high bioactivity of 45S5 is attributed to the later addition of 6% Phosphorus pentoxide (P2O5) by weight.[1]

Bioresorbable
These are the materials that degrade in the body while being replaced with regenerating tissues.[2]
Hydroxyapatite (Ca10(PO4)6(OH)2)
Hydroxyapatite can chemically bond rapidly with bone due to chemical similarities, and was first used in the 70’s before being accepted as an implant material in North America in 1988. It supports bone growth without degrading, but has a mechanical strength incapable of performing long-term load bearing. The applications of hydroxyapatite include metal coatings and porous blocks or beads to fill bone voids.[1]
Calcium Phosphate
Compared to hydroxyapatite, Calcium phosphate is easily absorbed into the body. Calcium phosphates are known for their porosity, and normally bond to bones through an apatite layer.[2] Examples of Calcium Phosphate compounds are Amorphous calcium phosphate (ACP), Dicalcium phosphate (DCP, CaHPO4), and Tricalcium phosphate (α-TCP, Ca3(PO4)2.[1] Calcium phosphates are used in skin treatments, dental implants, orthopedics, and more.[2]
Bioinert
Ceramics that retain structure after implantation and do not induce an immunologic response in the host.[2]
Alumina (Al2O3)
Highly inert, especially under physiological conditions, and has a corrosive resistances. It also has excellent wear resistance and hardness. Has dental applications, function as vertebrae spacers and extensors.[1][2] The body normally reacts to alumina by forming non-adherent fibers around the implant.[1]
Zirconia (ZrO2)
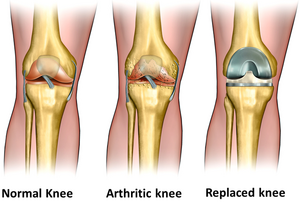

Zirconia is inert under physiological conditions like Alumina. Partially stabilized Zirconia (PSZ) has a higher flexural strength, toughness, reliability, and a lower Young’s modulus. Zirconia is good for long-term clinical use.[1] It is widely used in total hip replacement (THR), and as a replacement for knees, tendons, ligaments, and teeth. [2] Examples of Zirconia based bioceramics include Yttrium Stabilized Tetragonal Polycrystalline Zirconia (Y-TZP), and Zirconia/Alumina composites.[1][6]
Issues of Y-TZP
Biomedical grade Y-TZP had the best mechanical properties of the bioinert ceramics investigated, and quickly became a standard for hip and knee replacements. In 2001, patients with Y-TZP implants reported that the implant was failing, revealing a downside to Y-TZP. It was found that due to its meta-stability, it is prone to low temperature degradation in the presence of water, which triggers a progressive aging that eventually results in surface roughening and micro-cracking. The micro-cracks eventually cause more surface defects and lead to delayed failure of the implant. [3][4]
For dental applications, Y-TZP was also proven to lack stability in an oral environment in long term in vivo studies. In-vitro studies performed also suggested that aging might be an issue with Y-TZP used in an oral environment.[3][4]
Pyrolytic Carbon
Pyrolytic Carbon are brittle and do not perform well in load bearing applications, but do not suffer from fatigue.[2] It is commonly used in heart valves due to high strength, wear resistance, durability, and thromboresistance, or resistance to blood clotting. It can also be used for spinal inserts.[1]
Future of Bioceramics
The end goal of the future of bioceramics is regenerative medicine, including tissue regeneration without biomaterial. Other end goals are also tissue engineering assisted by bioactive scaffolds, minimally invasive implants, and conventional prostheses.[3]
Zirconia Toughened Alumina (ZTA)
As mentioned in the ‘issues with Y-TPZ’, its long-term stability is a current issue that needs fixing, specifically the degradation caused by water. This is being addressed by attempting to minimizing the oxygen-zirconia interactions. Within 5-10 years, Alumina-Zirconia composites might be used commercially. These are alumina reinforced zirconia composites, or ‘zirconia-toughened alumina (ZTA)’. The presence of the Alumina matrix stabilizes the Zirconia by essentially removing the Yttria that provides the oxygen with vacant sites for interactions. ZTA currently looks more promising than Y-TZP in physical properties. It significantly improves aging and cracking resistances, however, the improvements to the physical properties have currently stagnated and have no room for further optimization.[3]
Ceria and Magnesia doped Zirconia
When Y-TZP was first developed in the 80’s, different dopants and systems had also been introduced, namely Ceria and Magnesia doped Zirconia (Ce-TZP and Mg-PSZ), but Y-TZP was preferred for orthopedic implants due to its high strength. Ce-TZP and Mg-PSZ have been found to be competitive with Y-TZP in toughness and strength, and are being investigated for commercial release. Currently, Mg-PSZ is available for use in femoral heads in total hip replacements.[3]
Nanostructured Ceramics and Composites
Currently, most implantable biomaterials are developed in the micro-scale and the nanoscale is of interest. Considering current inert bioceramics, a higher hardness and wear resistance are expected if nanostructured ceramics are successfully developed, but the wear resistances of commercially available ceramics is sufficiently high. Developing nanostructured ceramics is incredibly difficult given that current microstructured ceramics have stability issues. Most of the effort on nanostructured ceramics is focused on improving the mechanical properties and designs of the implants.[3]
Two different nanocomposites are being researched: Alumina rich nanocomposites and Zirconia rich nanocomposites. The Alumina rich nanocomposites are composed of micro Alumina grains with Zirconia nanoparticles evenly dispersed inside, and the Zirconia rich nanocomposites is a nano-nanocomposite composed of phases smaller than 500 nanometers. Both attempt to increase crack resistance, tensile strength, and stability. Although they would theoretically improve the mechanical properties of the implants, they are extremely difficult to create. [3]
Non-oxide ceramics
Non-oxide ceramics are almost insensitive to crack growth and have overall higher structure reliability. They are also wear resistance and are perfect for bearings in biomedical implants. Currently, Amedica Corp. has non-oxide ceramics undergoing clinical trials. Reasons for their current commercial unavailability are due to the dominance of Y-TZP and the effort involved in manufacturing these pieces. They require to be sintered, or compacting without liquefying, at extremely high temperatures.[3]
Organic-Inorganic Bone Substitutes
Due to the brittleness of bioglasses and calcium phosphates and their inability to perform load bearing actions, a load bearing bioimplant is highly needed. Polymer-ceramic composites are ideal for this as they are less brittle while being tougher. The integration of polymer matrices is the main focus of this research, such as resorbable porous composite scaffolds. This is a fairly new field that is in its early stages and has issues such as how to incorporate the organic phase with the mineral phase.[3]
Biomimetric Approach
This part of current ongoing research deals with attempting to mimic the structure of an organisms parts such as a bone and nacre. Nacre is what covers the inside of the shell of mollusks and makes up a pearl's exterior.[3]
References
[1]Jayaswal, G.; Dange, S.; Khalikar, A. Bioceramic in Dental Implants: A Review. J. Indian Postho. Soc. 2010, 10,8-12.
[2]Patel, N.; Piyush, G. A Review on Biomaterials: Scope, Applications & Human Anatomy Significance. Int. J. Emerging Tech. Adv. Eng. 2012, 2, 2250-2459
[3] Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Euro. Ceram. Soc. 2008, 29, 1245-1255
[4] Chevalier, J.; Gremillard, L.; Deville, S. Low-temperature degradation of zirconia and implications for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1-32
[5] Cao, W.; Hench, L. Bioactive materials. Ceram. Int., 1996, 22, 493-507.
[6] Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biom. 1997, 1-25
[7] Ramalingam, M. Tissue engineering and regenerative medicine: a nano approach; CRC Press; Boca Raton: FL, 2012
[8] Macdonald, N; Bankes, M. Ceramic on ceramic hip prostheses: a review of past and modern materials. Arch. Orthop. Trauma Surg. 2014, 134, 1325-1333
